
By Elze van Hamelen
“Who controls the food supply controls the people; who controls the energy can control whole continents; who controls money can control the world.”
~ Henry Kissinger, 1973
I. Introduction
A New Chapter in the Great Poisoning
Something big is happening with food. The emergence of what I call “pharma food,” terminology that Solari also is adopting, is a primary trend we all need to know about, for these synthetic concoctions—which represent a new and disturbing chapter in the Great Poisoning—are already showing up in grocery stores and in brokerage accounts. In this report, we focus primarily on lab-grown meat, which provides an instructive lens for understanding the wider agendas at play.
The food supply is already far from perfect and has long been a vehicle for the Great Poisoning. However, it seems clear that the Going Direct Reset accelerated Mr. Global’s efforts to take over and transform food systems. In 2020, lockdown policies successfully bankrupted restaurants, coffee shops, and bars around the world, and now, with engineered spikes in energy prices, the bakeries and butchers that give local neighborhoods their distinctiveness are also disappearing. Meanwhile, we are witnessing the rise of highly centralized, controlled, and artificial alternatives run in part by AI and robots, ranging from production of artificial “meat” and “dairy” in large bioreactors, to insect farms, to vertical mega-factories making gene-edited produce indoors.
Synthetic meat is also where the control agenda meets the transhumanist agenda. At its core, the push to develop lab meat and other pharma foods is a quest to outdo nature and create life in a petri dish. The knowledge acquired by investing in lab-grown meat serves the broader transhumanist goal of “programming” cells and creating a new, artificial biology. By gaining an understanding of the processes that influence cellular rejuvenation and senescence, transhumanists’ ultimate aim is to extend the human life span—at least for billionaires.
In fact, the brave new synthetic food market is emerging with the help of generous billionaire funding and governments willing to act as bureaucratic units on behalf of a wider United Nations (UN) agenda. Watching big corporations and governments as well as nongovernmental organizations (NGOs) openly collaborate to change global and local “food environments” provides a case study into how the managerial side of the global governance structure operates.
What does the tsunami of fake foods mean for you? It means that working to ensure and protect food independence—at the personal, community, state, and other levels—has never been more vital. Doing so will help nourish and build successful families and communities. Maintaining your own and your family’s health by eating natural, fresh, poison-free foods may be one of the most important acts of resistance you can take in the times to come.
In this report, we will:
- Part I: Explain what we mean by “pharma food” (also see “Glossary of Terms”) and outline the environmental cover story being used to establish a centralized food system.
- Part II: Examine the flood of venture capital and billionaire investments being directed at the lab-grown meat and “alternative protein” sectors—despite a notable lack of consumer interest.
- Part III: Trace the history of lab-grown meat and how research, funding, and marketing have evolved over time.
- Part IV: Review the range of biopharmaceutical and transhumanist tools and technologies now being applied in the synthetic food sector.
- Part V: Describe the manufacturing process for lab-grown meat—and its many challenges.
- Part VI: Discuss the lack of attention paid to lab-meat safety and nutrition.
- Part VII: Consider global policies and agreements that are laying the groundwork for the pharma food agenda.
- Part VIII: Give examples of strategies being used to “nudge” consumers and investors toward pharma foods.
- Part IX: Describe the regulatory and cultural hurdles facing the lab meat industry.
- Part X: Address unanswered questions—why is there such an aggressive push to globally centralize food?
- Part XI: Review actions you can take to protect food independence and local food systems.
- Part XII: Emphasize the importance of taking the pharma food tsunami seriously and protecting you and your family.
What Is Pharma Food?
The trendy and newspeak-sounding “alternative protein” label is a catch-all term covering newfangled plant-based proteins as well as alt-protein sources such as insects and cell-cultured (lab-grown) meat. Regarding fake meat, it is hard to imagine anything more synthetic or artificial than cell-cultured meat produced using processes and technologies normally applied to the manufacture of biopharmaceuticals, including stem cells, regenerative medicine, genetic manipulation, synthetic biology, nanotechnology, and precision fermentation. This merger of technology with food—called “FoodTech” in Silicon Valley jargon or cellular agriculture (“cell-ag”)—refers to a vast set of technocratic activities mobilized to replace existing food systems.
Terminology containing the words “agriculture” and “fermentation” has a familiar and safe ring surely intended to minimize consumer suspicions about “novel” foods. However, technologies such as precision fermentation have very little to do with the traditional fermentation processes that humans have used for thousands of years to preserve foods and improve nutritional content. Fake meat, too, goes by a variety of names designed to persuade—though thus far, manufacturers and marketers largely have failed to come up with terminology that consumers find compelling. Some of the terms used to refer to lab-grown meat include:
- Synthetic meat
- In vitro meat
- Cultured (or cell-cultured) meat
- Cellular meat
- Clean meat
- Guilt-free meat
- Cruelty-free meat
- Slaughter-free meat
In my view, “pharma food” is a more appropriate term for these highly synthetic products. As I will explain in Part IV, “cell-ag” borrows its science and techniques directly from the pharmaceutical and medical industries. Insiders admit as much, noting that “cultured meat scientists are primarily biomedical … with expertise built in the healthcare industry around cell culturing, tissue engineering, and bioreactor design.”1
Glossary of Terms
Alternative protein: Alternatives to meat, such as plant-based burgers, lab-grown meat, and insects.
Biopharmaceuticals: Drugs derived from biological sources (organisms or living cells), including vaccines, hormones, monoclonal antibodies, and therapeutic enzymes; “biopharma” also refers to the industry that makes these products.
Bioreactor: A large vessel or vat in which biochemical processes are conducted; uses include the biotechnological production of pharmaceuticals and lab-grown meat.
Biotechnology (“biotech”): Technology based on biology; the integration of natural and engineering sciences to make a product or process.
Cellular agriculture (“cell-ag”): The production of agricultural products using cell cultures.
Cell culture: The maintaining and growing of cells in an artificial environment.
Cell line: A subcultured cell culture that can proliferate over longer time periods; produces standardized results.
Culture medium: Substance in which cultured cells grow.
Fetal bovine serum: A product made from the blood of calf fetuses.
FoodTech: A vast set of technocratic activities mobilized to replace existing food systems.
Gene editing: Genetic modification techniques that make targeted cuts in DNA; these “small” changes to the DNA are supposed to allow for the emergence of new traits into the organism.
GMO 2.0: A term used by organic and GMO watchdog groups to warn the public about the flood of new genetically modified products stealthily entering our environment and food supply.
Molecular farming: The genetic modification of plants to produce targeted substances, such as animal DNA; also referred to as “synbio on steroids.”
Nanotechnology: Technology that works at an incredibly small scale; a nanometer is one billionth of a meter and one millionth of a millimeter.
Neophobia: Fear of novel foods.
New breeding technique: See gene editing.
New genomic technique: See gene editing.
Novel foods: Foods and food ingredients (such as insects and nanomaterials) that most people historically would not recognize as food.
Precision fermentation: Genetically manipulated bacteria that excrete “milk,” “butter,” and other proteins.
Regenerative medicine: Studies the regeneration and senescence processes of cells, tissues, and organs.
Stem cells: Cells in an undifferentiated state that have the potential to differentiate into other cells of the body; when cultured, they have the capacity to proliferate quickly.
Synthetic biology (“synbio”): An engineering approach to biology used to create, design, and build functions in a cell.
Tissue engineering: A technique that tries to make cells from cell cultures adhere to “scaffolds”; the term is often used interchangeably with “regenerative medicine.”
List of Acronyms
CDC: U.S. Centers for Disease Control and Prevention
DARPA: Defense Advanced Research Projects Agency
DOD: U.S. Department of Defense
EFSA: European Food Safety Authority
ESG: Environmental, Social, and Governance (investment framework)
FBS: Fetal bovine serum
FDA: U.S. Food and Drug Administration
GFI: Good Food Institute
GMO: Genetically modified organism
GMP: Good Manufacturing Practice
GRAS: Generally recognized as safe
IARPA: Intelligence Advanced Research Projects Activity
NBT: New breeding technique
NGO: Nongovernmental organization
NGT: New genomic technique
NIH: National Institutes of Health
SDGs: Sustainable Development Goals
SPAC: Special purpose acquisition company
UNFSS: United Nations Forum on Sustainability Standards
USDA: U.S. Department of Agriculture
VC: Venture capital
WHO: World Health Organization
Environment as Cover Story
To build consumer demand for alternative proteins, governments, companies, and investors have mounted an aggressive and multifaceted sales pitch. The essence of their narrative is that traditional food production is extremely harmful and that this problem can only be solved through a major technocratic overhaul of the food system. Bill Gates famously summarized the agenda in early 2021, stating, “[A]ll rich countries should move to 100% synthetic meat.”2,3
Proponents make the claim that alt-proteins will strengthen global food security and solve the world’s food problems. The environment is also a prominent feature of the narrative, which asserts that alternative proteins will:
- Reduce carbon emissions
- Reduce water and land use
- Lessen biodiversity loss
- Diminish use of antibiotics and animal suffering
Last but not least, the promoted narrative is promising the pandemic-weary public that meat alternatives will decrease the risk of zoonotic diseases—infectious diseases that reportedly jump from animals to humans.4,5 In this context, alternative proteins are a cornerstone of the UN’s “One Health” approach, which has been steadily extending the global biosecurity agenda to target farming and all nature.6
Conveniently for globalists, their promotion of alternative proteins does not distinguish between traditional forms of smallholder farming—capable of solving all of the above-listed problems in a low-tech way—and large-scale industrial agriculture, which indeed does cause considerable damage.
II. Synthetic Meat and Alternative Protein Investments
Venture Capital Dollars … But No Sense?
On the surface, investing in synthetic meat would seem to have little to recommend it. Lab-produced proteins require high capital investments yet offer low profit margins and limited forecasts of hockey stick growth. Nor is the synthetic meat market a response to consumer demand.
Nevertheless, over the past decade, lab-grown meat companies have attracted large and growing infusions of money from venture capital (VC) firms and billionaire advocates such as Jeff Bezos, Bill Gates, and Richard Branson (see Table 1 and Figures 1-3).7 In fact, there are VC firms (such as New Crop Capital, Stray Dog Capital, and Kale United) that cater specifically to early-stage start-ups developing “animal-food substitutes.”8 Venture capitalists profess that their motivation for financing as-yet unprofitable synthetic meat products is because they want to solve “big ‘world problems.’”8
Table 1. Leading Pharma Food Companies and Investors
| Company & Year Founded (Location) | Funding to Date (Crunchbase) | Investors | Known for |
| Mosa Meat: 2013 (Maastricht, Netherlands) | $96M | Individuals: Sergey Brin, Leonardo DiCaprio, Jitse Groen Companies/Firms: Agronomics, Bell Food Group, Blue Horizon Ventures, DSM, M Ventures, Mitsubishi Corp | First burger |
| Upside Foods: 2015 (Berkeley, CA) | $606M | Individuals: Richard Branson, Bill Gates, Kimbal Musk, Jack Welch Companies/Firms: Cargill, Continental Grain, Norwest, SoftBank, Temasek, Threshold Ventures, Tyson Ventures | First on the market |
| Eat Just: 2011 (San Francisco, CA) | $465M | Individuals: Paul G. Allen (via Vulcan Capital), Marc Benioff, Heineken Family, Li Ka-shing, Vinod Khosla (Khosla Ventures), Jerry Yang Companies/Firms: Charlesbank Capital Partners, Founders Fund, Mitsui, Qatar Investment Authority, Temasek | First premarket approval by the U.S. Food and Drug Administration (FDA) |
| Aleph Farms: 2017 (Rehovot, Israel) | $119.4M | Individuals: Leonardo DiCaprio Companies/Firms: BRF, Cargill, Migros Group, Skyviews Life Science, Strauss Group, Thai Union Group, Temasek, VisVires New Protein | Cultured meat in space |
| Future Meat: 2018 (Rehovot, Israel) | $387.8M | Companies/Firms: ADM Capital (Cibus Fund), Archer Daniels Midland, Bits x Bites, Emerald Technology Ventures, HB Ventures, Manta Ray Ventures, Menora Mivtachim, Nestlé research partnership, Rich Products Corp, Rich Products Ventures, S2G Ventures, the Sander Group, Tyson New Ventures, Unternehmensgruppe Theo Müller, Yissum | One of the first to build a pilot plant Supplier of cell lines and other inputs for synthetic meat manufacturers |
| Meatable: 2018 (Delft, Netherlands) | $172.8M | Individuals: Taavet Hinrikus, Rick Klausner (formerly of Bill & Melinda Gates Foundation), Jeffrey Leiden, Bill Maris (founder of Google Ventures and Section 32) Companies/Firms: Agronomics, BlueYard Capital, DSM Venturing, Humboldt | Working with DSM to develop cell-culture media |
Figure 1: Alternative Protein Investors, by Type of Investor
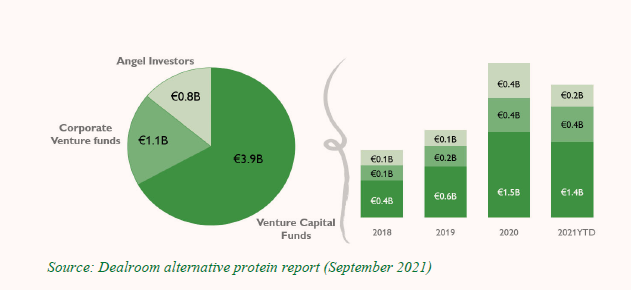
Figure 2: Venture Capital (VC) FoodTech Funding, 2016–2021 (USD billions)
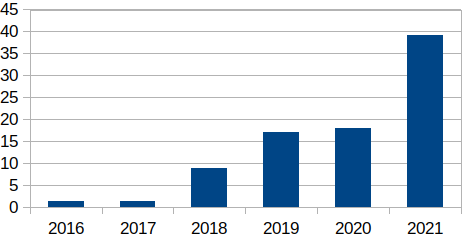
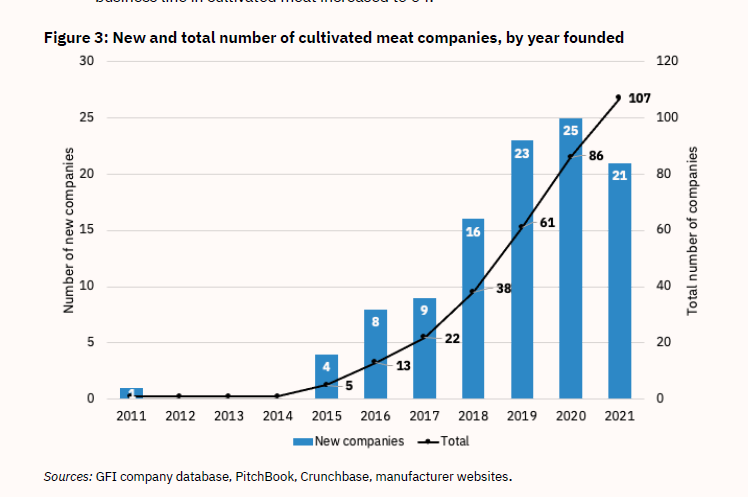
Figure 3: New and Total Number of Cultivated Meat Companies, by Year Founded
Investors appear to be betting that lab-grown meat will take off.9 Global management consulting firm McKinsey forecasts that the market for lab-grown meat could grow from $2 billion in annual sales to $20 billion or even $25 billion by 2030, “if consumers take to these products.”10 By way of comparison, however, the global meat sector was valued at $897 billion in 2021 and is expected to grow to $1354 billion by 2027.11
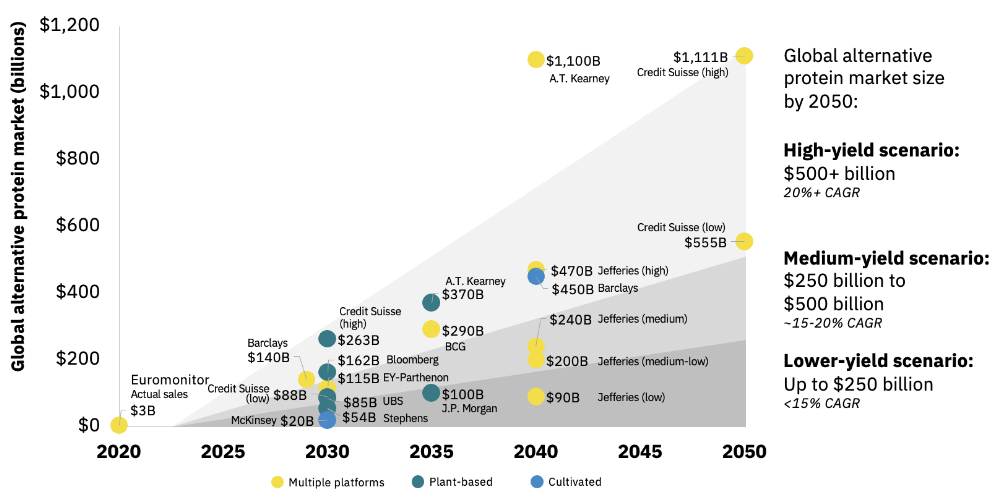
The Kearny management consulting firm has summarized multiple forecasts for the entire alternative protein market between now and 2050 (see Figure 4). The predictions vary widely, but what these forecasts show is that governments, FoodTech companies, and NGOs are serious about replacing real meat with non-meat alternatives through tightly controlled food systems.
Figure 4: Alternative Protein Market Forecasts
Consumers Say No
Exuberant market forecasts aside, consumer preferences constitute a major obstacle for synthetic meat. Confirming the public’s lack of interest in fake meat, a survey by Michigan State University involving 2,100 people across America found that only one-third reported being likely to “purchase foods that look and taste identical to meat, but are based on ingredients that are produced artificially.”12 Other survey results have varied depending on the wording chosen to describe the “meat” (see Figure 5).
Figure 5. Consumer Acceptance of Lab-Grown Meat: Survey Results
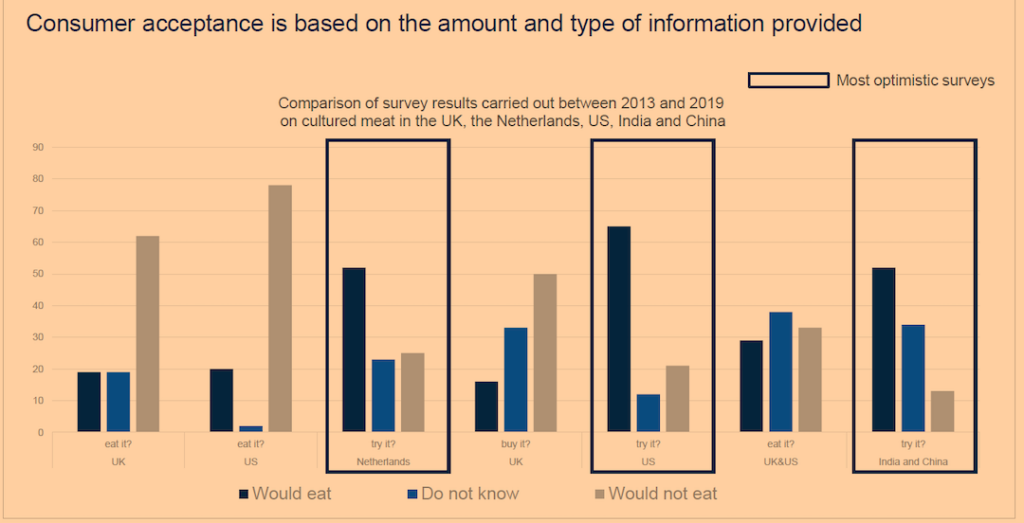
Although alt-protein manufacturers tested the waters years ago with items like “veggie burgers” and soy and almond milks, those products did not come with the promise of tasting like the real thing. Now, with new FoodTech tools at their disposal, the makers of plant-based items such as the Beyond and Impossible burgers are claiming just that—that their hyper-processed products derived from pea protein isolates and genetically modified soy look and taste like real meat. Thus far, health-conscious consumers have not taken to the unpalatable products or their off-putting ingredients.13
The scientific literature has a term for people who are not excited about eating cell-cultured foods and other FoodTech products—“neophobia” (fear of novel foods)—characterizing it as concern about “perceived unnaturalness,” dislike of “tampering with nature,” and “social distrust of the food industry.”14-16 The thinking goes that if manufacturers use cells from mice, rats, hamsters, or Japanese quail instead of cell lines from more commonly eaten livestock, such as cows or chickens, this may make the “neophobic” consumer more hesitant to try a lab burger.14
In 2022, consumers and ordinary investors sent a strong market signal: the stock value of Beyond Meat dropped by 77.5%. And in October 2022, meat giant JBS divested its plant-based businesses, although the company still has major investments in lab-cultured meat. It is unclear why JBS expects that consumers would choose lab burgers over plant-based burgers.17
The Going Direct Reset Speed-Up
As Table 2 shows, investments in pharma food alternative proteins accelerated noticeably during the Going Direct Reset. According to the Good Food Institute (GFI)—an NGO that actively promotes meat alternatives—precision fermentation start-ups had attracted $2.81 billion over the 2013–2021 period but saw a 285% increase in invested capital in 2021 compared to 2020.18 Likewise, while the lab-grown or “cultivated” meat sector drew combined funding of $1.93 billion for 2016–2021, it received $1.36 billion in 2021 alone—amounting to a 336% year-over-year increase.19
Table 2. Alternative Protein Market: Funding, by Sector18-21
| Sector | Funding Period | Combined Funding (USD or EUR) |
| Plant-based meat, egg, and dairy companies | 1980–2021 2021 | $6.36 billion $1.93 billion |
| Precision fermentation technology (alt-protein sector) | 2013–2021 2021 | $2.81 billion $1.69 billion |
| Lab-grown meat and seafood | 2016–2021 2021 | $1.93 billion $1.38 billion |
| Insect-based: For human consumption Insect-based: For animal consumption | As of 2021 As of 2021 | € 62 million € 1.2 billion |
Involvement of SPACs
Trend watchers such as FoodHack (a platform that “highlights and connects food entrepreneurs and innovators”) have observed that special purpose acquisition companies (SPACs)—blind pools that allow private companies to go public with less regulatory scrutiny—are a particularly useful vehicle for FoodTech companies that require large up-front investments.22
FoodTech companies acquired through SPACs include several indoor farm companies—Aerofarms (for $1.1B), AppHarvest ($1B), and Local Bounti ($1.1B).23 Ginkgo Bioworks, a platform for precision fermentation, went public in a $15 billion SPAC deal described as “one of the largest SPAC mergers to date.”24 Prominent Ginkgo Bioworks investors include Bill Gates and the Defense Advanced Research Projects Agency (DARPA); DARPA is the advanced-technology branch of the U.S. Department of Defense (DOD). Moolec Science, a company that genetically modifies plants so they produce animal proteins, also will go public through a SPAC.25
Current Status
In December 2020, Singapore became the first nation in the world to approve the sale of “cell-meat” products,26 permitting Eat Just’s lab-grown “chicken nuggets” to be sold by “retail, food service, hawkers, you name it.”27 At present, the San Francisco-based company sells its fake chicken product at a loss, asserting that “scaling production up to where it’s profitable ‘will take another three to six years.’”26
Although no other countries or companies had initiated sales of lab-grown meat to consumers as of December 2022, many precision-fermented creations—sold as “animal-free” dairy, egg, or meat products—are already on the U.S. market. Eat Just’s plant-based egg alternative is available at outlets like Whole Foods.28 Critics, characterizing these products as “GMO 2.0” or “new GMOs,” have expressed concern about the lack of adequate labeling.29,30
III. Getting Lab Meat Off the Ground
Visionary or Advance Guard?
The earliest known mention of the possibility of lab-grown meat was made by none other than Winston Churchill in 1931. Although his timeline turned out to be too optimistic, he was quite visionary about where biotech activities (which were not yet on the map) would lead—to people living in cities, and to food production behind closed doors. Churchill’s remarks are worth quoting at length:
“Even without the new sources of power [energy, ed.] great improvements are probable here. Microbes, which at present convert the nitrogen of the air into the proteins by which animals live, will be fostered and made to work under controlled conditions, just as yeast is now. New strains of microbes will be developed and made to do a great deal of our chemistry for us. With a greater knowledge of what are called hormones, i.e. the chemical messengers in our blood, it will be possible to control growth. We shall escape the absurdity of growing a whole chicken in order to eat the breast or wing, by growing these parts separately under a suitable medium. Synthetic food will, of course, also be used in the future. Nor need the pleasures of the table be banished. That gloomy Utopia of tabloid meals need never be invaded. The new foods will from the outset be practically indistinguishable from the natural products, and any changes will be so gradual as to escape observation.
If the gigantic new sources of power become available, food will be produced without recourse to sunlight. Vast cellars in which artificial radiation is generated may replace the cornfields or potato-patches of the world. Parks and gardens will cover our pastures and ploughed fields. When the time comes there will be plenty of room for the cities to spread themselves again.” 31
Given the lack of transparency in synthetic food labeling, Churchill’s comments about synthetic foods being introduced “so gradual[ly] as to escape observation” seem especially prescient.
Early Research—From Space to Art to Burgers
It was in the early 1950s that Dutch researcher Willem van Eelen, the “Godfather of cultured meat,” first posited the technical possibility of lab-cultured meat. Four decades later, in 1994, he filed the first lab-meat patent—the first of many.32 Van Eelen later put together a consortium of researchers and helped them attract €2 million in funding from the Dutch Ministry of Economic Affairs and food companies and universities for research conducted between 2005 and 2009.8,33
Meanwhile, in 2000 and 2001, the American space agency NASA funded a project to explore the possibility of growing cells into meat as a way to produce food during long-term space travel. The project was executed by a research team under the leadership of Morris Benjaminson at Touro College in New York. Apparently, Benjaminson had experience in securing NASA funding. The team cultivated goldfish cells, and the end product was deemed “acceptable as food.”8,34
Also in 2000, Harvard Medical School developed a creepy “art” project, culturing frog cells into a meat product; in 2003, the result was eaten during the “Disembodied Cuisine” exhibit at L’Art Biotech in France, even as the living frogs that had supplied the cells hopped around on the table. This “provocative work sought to engender discussion about the transgressive status of the tissue.”8
In subsequent years, several universities—in some cases, thanks to industrial contracts—researched “in vitro meat.” In general, however, researchers faced challenges in acquiring funding. New Harvest, a donor-funded NGO founded in 2004 that advocates for public investments in synthetic meat, was pivotal in turning this area of research from an obscure side project into a major field of its own, securing research grants and organizing various foundational conferences in the first decade of lab meat. According to the NGO’s current CEO, Isha Datar, “New Harvest literally buil[t] the field of cellular agriculture from scratch.”35
New Harvest’s founder Jason Matheny, according to the NGO’s Wikipedia page, “became interested in cultured meat after researching infectious diseases (HIV prevention) in India for a master’s degree” at the Johns Hopkins Bloomberg School of Public Health.36 Matheny’s research was part of a project supported by the Bill & Melinda Gates Foundation. Another source writes that Matheny’s inspiration for in vitro meat came from NASA’s goldfish project.8 Matheny has held a wide range of intelligence agency and national security positions, becoming president and CEO of RAND Corporation in July 2022 (see “Profile of an Alt-Protein Booster”).
Lab-grown meat had its first major breakthrough in 2013. Mark Post of Maastricht University cultured the first lab-grown burger, tasted at a high-publicity event in London that attracted considerable media attention. Google’s co-founder Sergey Brin funded the burger to the tune of €250,000 ($330,000),37,38 and in 2015, the first lab-grown meat start-ups were launched. Their numbers took off in 2018, with over 20 new players per year in subsequent years.
Profile of an Alt-Protein Booster
Jason Matheny may have founded New Harvest, but his colorful resumé primarily illustrates his intelligence agency and national security connections. With several graduate degrees from Duke University and Johns Hopkins University (JHU), Matheny’s professional trajectory has included positions at Oxford University (where, as Director of Research at the Future of Humanity Institute, he explored “existential” and catastrophic risks facing humanity and their mitigation); the World Bank; JHU’s Applied Physics Laboratory; the Center for Biosecurity; and Princeton University. In 2009, Matheny joined the Intelligence Advanced Research Projects Activity (IARPA) within the Office of the Director of National Intelligence, where he held several positions, ending as Director.
In 2019, Matheny became founding director of a Georgetown University think tank, the Center for Security and Emerging Technology (CSET). Open Philanthropy, an NGO that also channels extensive funding to the alt-protein sector, made a series of generous awards to Matheny’s Center, including:
- $55 million to launch the think tank (January 2019)
- $8 million “for general support” (January 2021)
- $3.3 million to investigate “the extent and risks of dual-use research in the biosciences” (August 2021)
- That same month, a whopping $38.9 million “for general support” (August 2021)
In 2018, Congress appointed Matheny to serve on the National Security Commission on Artificial Intelligence. In early 2021, Matheny left CSET to lead White House policy on technology and national security, also serving as Deputy Director for national security at the Office of Science and Technology Policy. In mid-2022, he became RAND Corporation’s CEO.
Matheny has served on numerous public and private committees—for the National Academies, the Departments of Commerce and Energy, the American Association for the Advancement of Science, the Nuclear Threat Initiative, the Center for a New American Security, and the Carnegie Endowment for International Peace—focused in the areas of intelligence, national security, emerging technology, AI, machine learning, and other related areas. For his achievements, according to Wikipedia, Matheny received “the Intelligence Community’s Award for Individual Achievement in Science and Technology, the National Intelligence Superior Service Medal, and the Presidential Early Career Award for Scientists and Engineers.”
His Wikipedia page notes only in passing that “Matheny is recognized for having popularized the concept of cultured meat.” His resumé makes you wonder why kick-starting the field of lab-grown meat would be a priority of the U.S. intelligence community.
Sources:
- “Georgetown University—Center for Security and Emerging Technology.” Open Philanthropy, January 2019.
- Howard Fine. “Rand taps former White House policy staffer Matheny as next CEO.” Los Angeles Business Journal, Jun. 13, 2022.
- “Jason Gaverick Matheny.” Wikipedia. Accessed Nov. 15, 2022.
- “Jason Matheny” RAND Corporation. Accessed Dec. 7, 2022.
The Good Food Institute and Its Lab Meat Agenda
From the time of its founding in 2016, the Good Food Institute (GFI) has played a major role in pushing the alternative protein agenda (articulating its vision as “A world where alternative proteins are no longer alternative”). Where New Harvest mostly focuses on supporting lab-grown meat research, GFI—with the help of 100 team members across a U.S. office and five international affiliates—engages in a far broader set of activities. Table 3 summarizes key indicators of both organizations’ reach for the year 2021.
Table 3. Good Food Institute and New Harvest 2021 Scoreboard
| Indicators (2021) | Amount/Number |
| Good Food Institute (GFI) Research grants awarded Multidisciplinary members (entrepreneurs, investors, scientists) Open-access data/insights published (GFI teams, grantees, program partners) University campuses growing next-generation alt-protein innovators | $5.7 million 2,310 75 16 |
| New Harvest (NH) Funding Public cell-ag research funding secured by labs receiving New Harvest catalytic funding Donors Peer-reviewed articles published Contributions to academic grants High-impact projects (nonprofit, NGO, researcher, government collaborations) | $2.5 million $11.9 million 507 9 12 10 |
Sources:
- Seeding Radical Change: 2021 Year in Review. Good Food Institute, 2021.
- “New Harvest: We are the global nonprofit building the field of cellular agriculture.”
- Our 2021 Annual Report. New Harvest, Jun. 3, 2022.
GFI’s broad areas of intervention include:
- Helping start-ups, incumbent food companies, restaurants, and meat producers to develop alternative proteins
- Working with governments, including at the UN level, to assure regulatory approval of lab-grown meat
- Litigating for favorable labeling
- Conducting market research
- Organizing conferences
- Providing grants for research
- Identifying research opportunities
In the research realm, GFI explains in a strategic plan dated September 2021 that it helps facilitate funding by identifying available grants from organizations such as the “National Science Foundation, European Commission, U.S. Department of Agriculture, and U.S. Department of Energy, and private foundations, including the Bill & Melinda Gates Foundation, David and Lucile Packard Foundation, and Foundation for Food and Agriculture Research.”39
According to GFI’s website, all of its activities are “100 percent powered by philanthropy.”40,41 Although GFI’s “generous donors … wish to remain anonymous,” a quick Internet search reveals where some of its sources of funding are coming from, with at least $6.5 million donated by the “left of center” NGO, Open Philanthropy. Founded in 2017 by Facebook co-founder Dustin Moskovitz, Open Philanthropy funds numerous alt-protein initiatives, ranging from start-ups to research organizations. Holden Karnofsky, the co-CEO of Open Philanthropy, euphemistically stated in an interview with the New York Times that his NGO is “the largest funder in the world of farm animal welfare.”42 Interestingly, the organization’s action fund also financed multiple organizations affiliated with Black Lives Matter and, as noted in “Profile of an Alt-Protein Booster,” the Georgetown University think tank established by New Harvest founder Jason Matheny.
Y Combinator, a start-up accelerator, also has provided funding to GFI. Forbes described Y Combinator in 2012 as one of Silicon Valley’s most successful accelerators.43 Although it is known for its role in start-ups such as Airbnb, Quora, Dropbox, Coinbase, and Reddit, Y Combinator also provides seed funding for companies working in the areas of “synbio,” gene technology/genetic engineering, AI, regenerative medicine, longevity research, artificial embryo and artificial womb technology, and lab-grown meat.
A Global Phenomenon
Although most lab-grown meat investments thus far have originated in the U.S., VC firms in other regions are also increasingly active (see Table 4 and Figure 6).
Table 4. Leading Venture Capital Investors in Synthetic Meat and FoodTech, by Region
| Firm | Year Founded | Comments |
| United States Y Combinator Founders Fund Stray Dog Capital S2G Ventures Unovis New Crop Capital Breakthrough Energy Ventures | 2005 2005 2013 2014 2015 2015 2016 | Has helped launch more than 3,000 companies Co-founded by Peter Thiel Early and sustained focus on alt-proteins Focus on “entire food supply chain” “Reimagining the food industry” Co-founder Bruce Friedrich also a GFI co-founder Founded by Bill Gates |
| Europe M Ventures (Netherlands) BlueYard Capital (Germany) CPT Capital (United Kingdom) ProVeg Incubator (Germany) Kale United (Sweden) Agronomics (United Kingdom) Food Labs (Germany) Blue Horizon (Switzerland) | 2009 2016 2017 2018 2018 2018 2020 2020 | VC arm of Merck; offices in Germany, Israel, U.S. Focus on “programmable biology” “[R]eplacing animals in the supply chain” Plant-based and cultured food start-ups “Mak[ing] a dent in climate change” Focus on cell-ag and cultivated meat Alt-proteins, FoodTech technologies Alt-proteins, synbio, cell-based food |
| Asia ZhenFund (China) Bits x Bites (China) Lever VC (Hong Kong/U.S.) Cibus Enterprise Fund (Hong Kong) | 2011 2015 2017 2017 | Created in collaboration with Sequoia Capital China Cell-based meat, alt-proteins, agritech “Investing in the future of protein” (China Fund) Managed by ADM Capital: “agtech and foodtech” |
| Middle East Qatar Investment Authority The Kitchen FoodTech Hub Fresh Start (Israel) | 2005 2015 2019 | Participated in 2021 Eat Just $200M funding round “Cutting-edge” FoodTech start-ups Led by Israel’s largest food/beverage companies |
Figure 6: Venture Capital Investments in Lab-Grown Meat, by Region
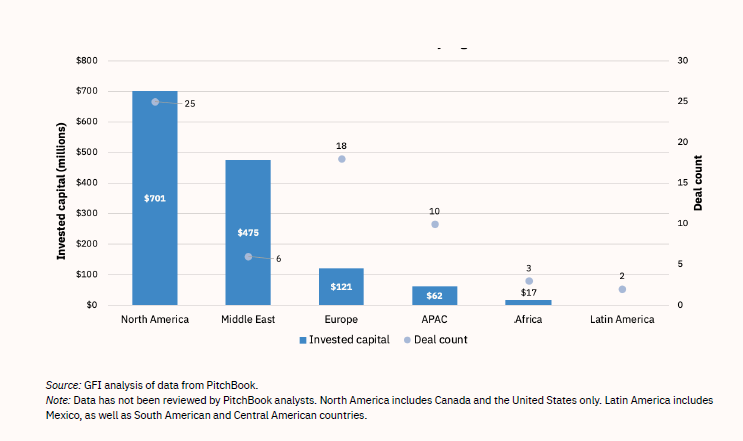
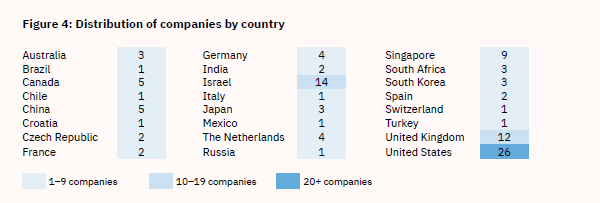
In 2021, Wired magazine pronounced Israel “a fake meat powerhouse.”44 As of 2021, Israel hosted 14 cultured-meat start-ups—a large number relative to the size of the country, and second only to the U.S.’s 26 lab-grown meat start-ups (see Table 5). To create a good investment and start-up climate, the Israeli government provides funds for two start-up incubators, Fresh Start and the Kitchen. Despite the investments and interest in lab-produced meat, Jewish and Muslim religious experts are not yet in agreement about whether the novel products are kosher or halal.44,45
Table 5: Lab-Grown Meat Start-ups, by Country (2021)
With limited agricultural capacity and high population growth, the Arab Gulf states depend on imports for 90% of their food and are interested in opportunities to increase self-sufficiency. Tariq Al-Wahedi, CEO of Agthia Group (a producer of animal feed located in the United Arab Emirates), explained to Arab News in 2019:
“Farming used to depend on land, but today many countries are relying on vertical farming. This is changing the concept of food security. It’s no longer about having reserves of food but about being on top of innovation and technology, and (being) able to foresee what’s coming in our role as industry leaders. Innovation now gives us an edge and a better life.”46
Eyeing the lucrative Gulf market, Josh Tetrick—CEO and co-founder of Eat Just—commented in the same Arab News article:
“Saudi imports about 50 percent of its meat from Brazil, which is incredibly inefficient for many reasons, and it’s not secure. It purchases land for its animals outside of Saudi and pays for security to protect that land. It would be better for it to secure its own food supplies and export modern technologies outside to the Gulf Cooperation Council and to Europe—why should it be reliant on other countries when it has the ability to innovate?” 46
The government of Qatar is also interested. In August 2021, it announced that the Qatar Free Zones Authority (QFZA) and Doha Venture Capital (DVC) had partnered with Eat Just to build the first lab meat production plant in the Middle East outside of Israel.47,48
In 2017, the Chinese government signed a $300 million trade agreement with Israel that positioned Israeli start-ups SuperMeat, Future Meat Technologies, and Meat the Future to supply China with lab-cultured meat.8 Although there are only five known Chinese lab meat start-ups currently, analysts expect the government to increase its investments in this area of research and entrepreneurial activity.49 In 2021, the government funded a three-year project focused on “High-efficiency biological manufacturing technology of artificial meat,”50 and it is funding research programs to support development of lab meat at China’s National Natural Science Foundation, the China Meat Food Research Center, and the Beijing Academy of Food Sciences.
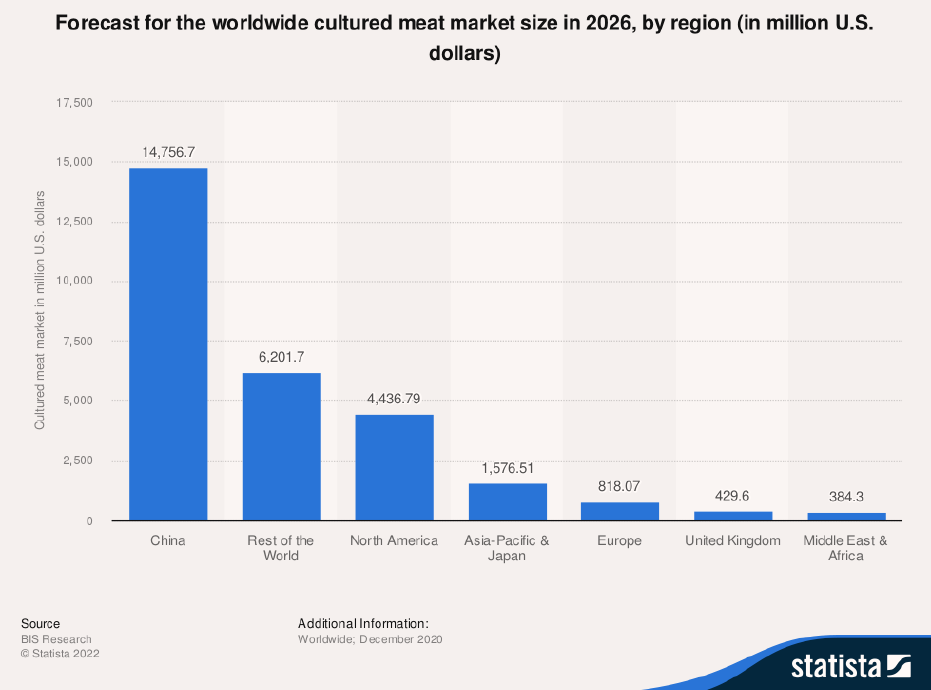
In fact, market researchers are predicting that China may become one of the largest players in the field of lab-grown meat (see Figure 7). China considers synthetic meat and other “foods of the future” an essential part of its food security policy, and development of meat alternatives is part of its most recent Five-Year Agricultural Plan, announced in early 2022.50
Figure 7: Cultured Meat Market Forecast for 2026, by Region
Enter the Meat Packers and Food Conglomerates
In 2017, incumbent meat-packing and meat-producing conglomerates joined VC firms and billionaires in investing in lab-grown meat.8 All of the world’s major meat producers now have alternative protein divisions. U.S.-based companies investing in lab-meat start-ups include:19,51
- Tyson Foods—the world’s second largest meat producer (Arkansas)
- Cargill—the world’s third largest meat producer (Minnesota)
- Smithfield Foods—the world fourth largest meat producer (Virginia-based subsidiary of China’s WH Group)
- Archer Daniels Midland—food and animal nutrition and commodities conglomerate (Chicago)
- Continental Grain—animal production and feeding (New York)
- Rich Products Corporation—frozen foods (Buffalo)
Companies supporting lab-grown meat start-ups outside of the U.S. include:
- PHW Group—Europe’s largest poultry producer (Germany)
- Danone Manifesto Ventures—VC arm of French multinational Danone (Paris and New York)
- SHV Holdings—parent company of animal nutrition company Nutreco (Netherlands)
- Thai Union Group—world’s largest canned tuna processor (Thailand)
- JBS—the world’s largest meat producer (Brazil)
- BRF—the world’s ninth largest meat producer (Brazil)
In 2021, both of Brazil’s meat-producing giants announced ambitious lab-grown meat ventures. JBS reported it was acquiring Spanish lab-meat company BioTech Foods for $41 million, in addition to launching a lab-meat research center in Brazil; meanwhile, GFI brokered a partnership between BRF and Israeli cultivated meat company Aleph Farms to enable large-scale production of lab meat, with plans to offer it in supermarkets by 2024.19
Other major food purveyors are not only investing in alternative protein offerings but are setting targets to shift their product portfolios. According to a report by the FAIRR Initiative (a coalition of institutional investors that stimulates divestment out of livestock farming by promoting ESG ratings), 28% of the largest food firms—including Unilever, Conagra, Nestlé, Tesco, and Sainsbury’s—have adopted such targets.52 Given that just a few big players dominate most food industry sectors and have inordinate influence over markets, research, and policy, it should give us pause that large meat producers and other food corporations are planning to shift their product portfolios in such a radical way.51
Scaling Up with Calls for “Infrastructure”
NASA’s experiment and the flashy tasting of a lab-grown burger in London established a proof of concept. Still, despite the flood of investments in start-ups, companies repeatedly missed their launch dates, slowing down the industry’s anticipated development.26 Apparently, moving from a proof of concept to large-scale production is not feasible without wider support, including knowledge from pharma and food incumbents, other scientific and technological innovations, regulatory support—and consumer acceptance.
In 2022, New Harvest CEO Isha Datar outlined the need for a synthetic meat “infrastructure” as follows:
“Before there was a cellular agriculture institute, textbook, or PhD program, there were more than 100 cellular agriculture companies around the world. If cellular agriculture is a city, it is one without public infrastructure. There are privately owned ‘houses’ popping up everywhere, but the roads, bridges, sewers, and public squares—all of which are crucial to a thriving ecosystem—are yet to be built. What is the likelihood of success for this emerging community without this critical infrastructure? Cellular agriculture must build up its open infrastructure to succeed.”53
With the launch of the Going Direct Reset, this infrastructure began quickly emerging.
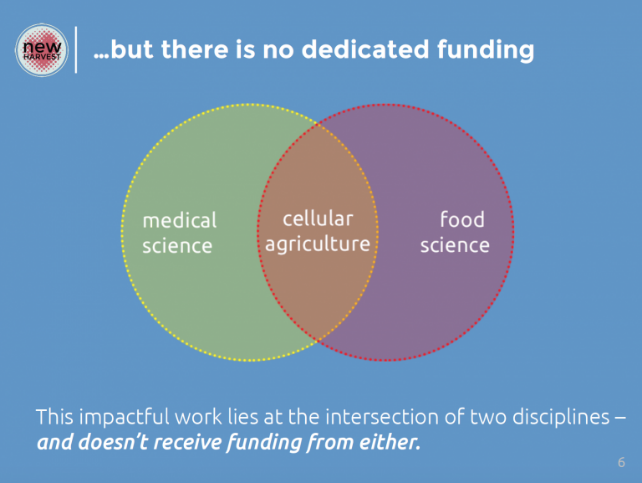
Private “Force Multipliers” and Public Funds
In a simple but illustrative Venn diagram, New Harvest’s Isha Datar describes cellular agriculture as the product of cross-disciplinary collaboration between food science and pharma science (see Figure 8). Similarly, the GFI notes that in addition to the one hundred-plus start-up companies trying to bring lab-grown meat to market, there are more than 60 companies—many of them from the biotech/life sciences sector—offering specialized products or services to the lab-meat industry.19 GFI writes: “This business-to-business (B2B) activity will be a valuable force multiplier for the industry, as these services and expertises will benefit multiple businesses rather than stay siloed in a single company.” Notable B2B examples include:
- A collaboration agreement between pharma giant Merck, Tufts University in the U.S., and Germany’s Technical University of Darmstadt to research design of the bioreactors used to make cultivated meat19
- A partnership between the Dutch life sciences corporation DSM and Dutch lab-meat company Meatable to develop a medium in which the cells can grow19
- Joint exploration by food giant Nestlé and Israeli firm Future Meat to identify possibilities for cultured meat54
Figure 8: Synthetic Meat at the Intersection of Food and Pharma Science
In late 2021, Datar boasted about how the starter funding provided by New Harvest for cultured meat research has paid off with a wave of research and “big time government grants.” Describing a lab at Tufts University, Datar stated:
“Since 2016, the Kaplan Lab has been our biggest investment in a single laboratory. With just under $1M, we supported six New Harvest grantees growing cell-cultured caterpillar steaks, fat, and nutritionally engineered beef. Along with their incredible Principal Investigator, Dr. David Kaplan, those grantees multiplied that initial funding ten fold.” 55
By Datar’s estimate, New Harvest “alumni” have raised $1.19 billion for private research and development and created over 600 industry jobs.
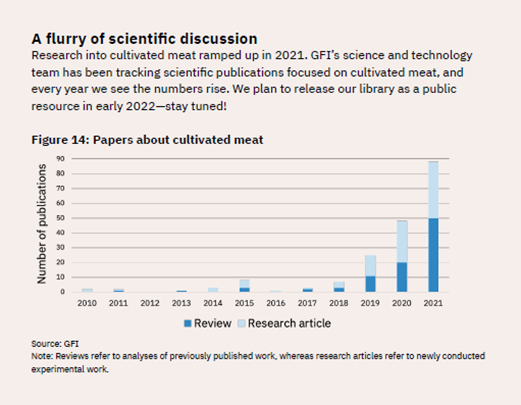
As another indicator of the explosion in research, scientific publications related to lab-cultured meat have skyrocketed, going from fewer than 10 in 2018 to nearly 90 in 2021 (see Figure 9).
Figure 9: Number of Scientific Publications on Cultivated Meat, 2010–2021
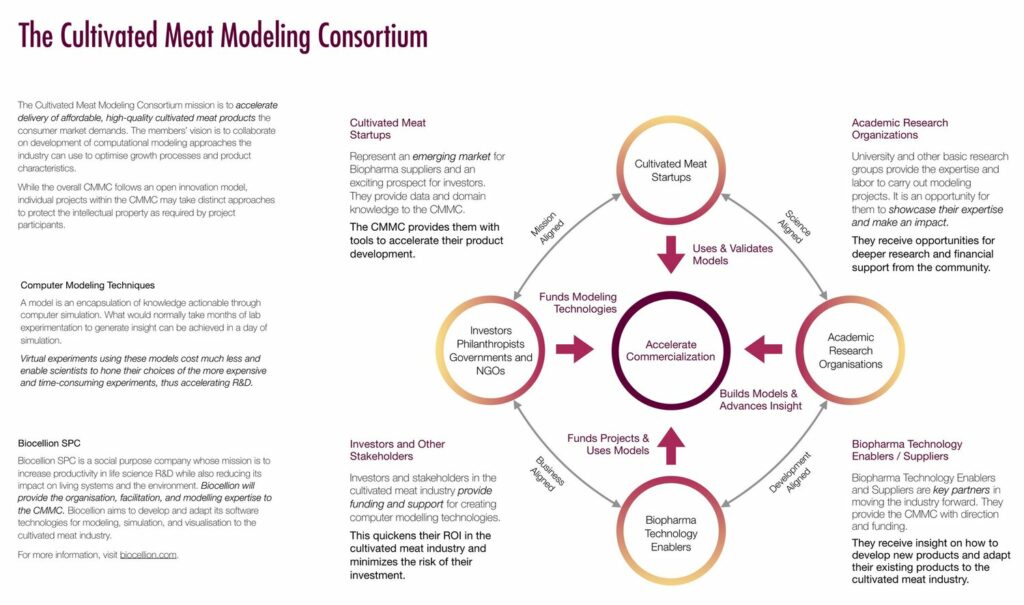
Over the span of just a few years, lab meat also has seen the launch of numerous consortia—involving public-private partnerships and exchanges between start-ups, food and pharma industry incumbents, universities, NGOs, and government representatives (see Figure 10 and “Lab-Grown Meat Consortia”). This environment provides ample opportunities to lobby for funding and legislative support.
Figure 10: Building a Cultivated Meat “Ecosystem”: The Cultivated Meat Modeling Consortium (CMMC)
In the Netherlands, for example, a Cellular Agriculture consortium secured €60 million in government funding in April 2022—the world’s “largest-ever public investment in the cellular agriculture field”—to create an “ecosystem” intended to attract synthetic meat start-ups and help them thrive.56 The coalition includes two universities (the world-renowned agricultural university in Wageningen and the technical university in Delft); food and life sciences corporations Nutreco and DSM; the Netherlands’ Sustainable Development Goal (SDG) organization; start-ups Mosa Meat and Meatable; and others. Recognizing that operating a biotech plant is not only capital-intensive but is knowledge- and skill-intensive, the group is joining forces to develop training programs to deliver qualified personnel to synthetic meat start-ups. The coalition is also working to inform the public at large about developments in the field and foster societal acceptance of these new “foods.”57
Lab-Grown Meat Consortia
Consortia and public-private partnerships promoting lab-grown meat include groups in most regions of the world; 11 of the 14 consortia were established in 2021 or 2022.
Global
- Cultivated Meat Modeling Consortium (CMMC), founded in 2019: CMMC connects research with pharma, NGOs, and start-ups to speed up time to market. No government funding.
- Global alliance for advancing cultivated foods, founded in 2022: A consortium of U.S., EU, and Asian cellular agriculture partners joining forces to advance synthetic meat. No government funding.
United States
- Association for Meat, Poultry and Seafood (AMPS) Innovation, founded in 2019: A collaboration platform to speed up time to market and the Food and Drug Administration (FDA) and U.S. Department of Agriculture (USDA) approval process. No government funding.
- National Institute for Cellular Agriculture, founded in 2021: Hosted at Tufts University, with $10M in funds from USDA.
- OpenCellAg, founded in 2022: A New Harvest project supporting a “network of excellence building data and tools for cellular agriculture.” No government funding.
European Union
- EIT Food, founded in 2019: An EU-funded pan-European network for innovation and technology in food. EU funding for agrifood projects (€56.4M), start-ups (€6.8M), food system education (€7.8M), innovation projects creating marketable products and services (€25.6M), and public engagement (€5.2M).
- Feed for Meat, founded in 2021: Research and development program of Nutreco and Mosa Meat, funded by EU (€2M).
- Cellular Agriculture Europe, founded in December 2021: Coalition of food companies.
- Cellulaire Agricultuur Nederland, founded in 2022: Consortium funded by Dutch government (€60M) to create an ecosystem that attracts synthetic meat start-ups.
- CellAg UK, founded in 2022: Platform to create a “cellular agriculture community in the UK.” No government funding.
Asia
- CulNet Consortium, founded in 2021: Open innovation platform in Japan. No government funding.
- APAC Society for Cellular Agriculture, founded in 2022: Asian synthetic meat ventures joining forces to develop ecosystem for “novel meats” in Asia. Based in Singapore. No government funding.
Other
- Alternative Proteins Council, founded in 2021: Representative group for the alternative protein sector in Australia and New Zealand. No government funding.
- Israeli cultivated meat consortium, founded in 2022: Consortium of 14 companies and 10 academic and research institutions, launched with $18M grant from the government’s Israel Innovation Authority.
Source:
“APAC, Europe, United States industry associations join forces in a global alliance for advancing cultivated foods.” Cellular Agriculture Europe, Oct. 24, 2022.
IV. Big Pharma—and Transhumanism—Show the Way
Scientific Foundations of Lab Meat
The production of lab-cultured meat builds on and brings together scientific techniques and processes first developed and applied in the pharmaceutical and biotech industries (now merged as “biopharma”). Other technologies belong to what we are calling the “transhumanist toolbox.”
These technological precedents include:
- Genetic engineering (see “Insulin: the First Genetically Engineered Drug”)
- Cell culturing (including the use of fetal cell lines and stem cells)
- Tissue engineering and regenerative medicine
- Gene editing
- Precision fermentation
- Synthetic biology
- Molecular farming
As already noted, organic and GMO watchdog groups loosely use the term “GMO 2.0” to encompass many of these technologies. These groups are working to warn the public about the flood of new genetically modified products that are stealthily entering our environment and food supply.
One more technology that is part of the transhumanist toolbox are “gene drives,” a technique that manipulates organisms in such a way as to spread a new genetic trait throughout an entire population in the wild.58 As far as we know, gene drives are not used for synthetic meat production.
Insulin: the First Genetically Engineered Drug
In 1982, the pharmaceutical industry began marketing the first genetically engineered drug: synthetic insulin. Production of synthetic insulin involves inserting the genetic code for human insulin into E. coli bacterial cells and fermenting the genetically modified bacteria to produce insulin. Leveraging this process, the use of genetically engineered organisms in the manufacturing of drugs and biologics has become a biopharma “industry standard.”
As Table 6 shows, the markets for cells, cell lines, cell culture media, tissue engineering, and other products supporting biopharma—and lab meat—are all immense.
Table 6. Market Size of Sectors Supporting Biopharma
| Input / Supply | Market Size (as of year) |
| Primary cells | $970 million (2020) |
| Primary cells—global market | $1.3 billion (2022) |
| Stem cells | $6.87 billion (2016) |
| Cell line development | $4.16 billion (2019) |
| Growth media | $4.9 billion (2021) |
| Tissue engineering | $16.9 billion (2021) |
| Biopharmaceuticals—global market | $403 billion (2022) |
Cell Culturing
Cell culturing refers to the propagation of human, animal, or insect cells in an artificial environment.59 Cells obtained directly from tissue become the “primary culture”; the first subculture of cells taken from a primary culture then becomes a cell line that can be used for further cell culturing. Cell lines can grow in a single layer or in a fluid called a “growth medium.”
Cell cultures and tissues are stored at public and private tissue banks for medical research, education, and tissue transplants. The world’s largest tissue bank, the American Association of Tissue Banks (AATB), stores over 4,000 cell lines.60
From scientists’ standpoint, primary cells—those taken directly from the body—have the disadvantages of a limited life span and a slow growth rate. However, some cell cultures can grow indefinitely; this is called an “immortal” cell line. These may occur naturally, such as when biopsies taken from some cancers exhibit this trait. For example, the human “HeLa” cell line, still widely in use, was derived in 1951 from the cervical cancer of a woman named Henrietta Lacks. It is also possible to create immortal cell lines through genetic manipulation.14
Life science and biomedical researchers consider cell lines among their most basic tools. Illustrating the importance of cell cultures for the global pharmaceutical sector, the industry grows 40% of its product pipeline in cell culture media.61 Cell culture applications include characterizing cancer cells, testing for toxicity, making cells for gene therapy, and producing drugs and vaccines.59 In fact, cell lines are used to produce the active ingredients in most vaccines,59 and many use human fetal cell lines.62,63 The significant amounts of cell slurry that are a waste product from the vaccine production process are the base material for lab-grown meat.64
Human Fetal Cell Lines
Although the biotech and pharmaceutical industries make frequent use of fetal cell lines, few among the general public understand what they are or are aware of their use. The topic attracted greater attention when Project Veritas aired a video of a Pfizer whistleblower in October 2021 (see “Pfizer Whistleblower Tells All”).65 Both before and after these disclosures, multiple well-researched articles in the new media have highlighted how fetal cell lines are created—by “harvesting” tissue from live fetuses:
- Caryn Lipson titled an article for America’s Frontline Doctors, “Aborted fetal cells and vaccines—a scandal much bigger than Pfizer’s whistleblower ever imagined.”66
- Christian Hacking, Public Engagement Officer for the Centre for Bio-Ethical Reform in the UK, wrote “What the HEK?!” in reference to the use of “a kidney from a dissected unborn baby” to create a much-used fetal cell line called HEK 293.67
- Jon Rappoport wrote a series of articles about medical research and “The Abortion Culture.”68
- LifeSiteNews set the record straight in “Vaccines using fetal tissue: 12 faulty assumptions.”69
- Robert F. Kennedy, Jr. conducted a stunning interview with biotech entrepreneur Dr. Theresa Deisher about aborted fetal DNA and vaccines.70
Pfizer Whistleblower Tells All
In October 2021, whistleblower Melissa Strickler released internal emails about Pfizer’s use of fetal cell lines for its Covid-19 injections. Pfizer’s Senior Director of Worldwide Research, Vanessa Gelman, confirmed, “One or more cell lines with an origin that can be traced back to human fetal tissue has been used in laboratory tests associated with the vaccine program.”
Gelman also wrote to colleagues, “We have been trying as much as possible to not mention the fetal cell lines,” adding:
“From the perspective of corporate affairs, we want to avoid having the information on fetal cells floating out there…. The risk of communicating this right now outweighs any potential benefit we could see, particularly with general members of the public who may take this information and use it in ways we may not want out there. We have not received any questions from policy makers or media on this issue in the last few weeks, so we want to avoid raising this if possible.”
Source:
“PFIZER LEAKS: Whistleblower goes on record, reveals internal emails from chief scientific officer & senior director of worldwide research discussing COVID vaccine … ‘We want to avoid having the information on the fetal cells floating out there.’” Project Veritas, Oct. 6, 2021.
In two of the most thorough investigations into this topic, researcher Monica Seeley verified some of the almost unbelievable claims about live harvesting.71,72 Seeley extensively cited former vaccine researcher and biologist Pamela Acker, who explained, “Just as you can’t transplant a dead organ into a living body, you cannot make a cell line out of dead tissue.” According to Acker, the procurement of human fetal tissue “has to be done in a methodical kind of way in order to obtain the kind of tissue—live tissue—that will be successful for this kind of research.” Acker noted that through the mid-20th century, many primary sources from the medical literature were “surprisingly frank and open” about the methods in use—harvesting live tissue was “part and parcel of the medical research that was going on in the 1950s and 60s.”71
Seeley confirmed Acker’s assertions with quotes by other past and present experts in the field, who indicated that for their purposes, not only do researchers need a fetus to be delivered alive but that they will readily adapt the delivery method to their harvesting goals.72 For example:
- Embryologist Dr. C. Ward Kischer, emeritus professor of anatomy at the University of Arizona College of Medicine, stated in 1958: “In order to sustain 95% of the cells, the live tissue would need to be preserved within 5 minutes of the abortion. Within an hour the cells would continue to deteriorate, rendering the specimens useless.”
- The late Spanish bioethicist Dr. Gonzalo Herranz commented: “[T]o obtain embryo cells for culture, a programmed abortion must be adopted, choosing the age of the embryo and dissecting it while still alive in order to remove tissues to be placed in culture media” [emphasis in original].
- The so-called “Godfather of vaccines,” Dr. Stanley Plotkin, also confirmed these facts when he was obliged to testify under oath in 2018.73
To make matters worse, Seeley’s investigation found that the fetuses “are generally not given any anesthetic, because that would disrupt the cells that the researchers are trying to extract.”71 Again, confirmation of these claims can be found in other sources; Ian Donald, who pioneered the use of ultrasound in obstetrics, reported that “experiments were being performed on near-term alive aborted babies who were not even afforded the mercy of anesthetic” to alleviate their suffering.74
Although descriptions of current practices are not as “open and frank” as they were in the 1950s, various sources indicate that the practice of keeping fetuses alive to “optimally” harvest tissue continues. An investigation into the U.S. abortion industry by the Select Investigative Panel of the Energy & Commerce Committee published in December 2016—chaired by Rep. Marsha Blackburn from Tennessee—reported that on multiple occasions, abortion procedures had been adjusted to obtain more fetal tissue.75,76
In 2021, Judicial Watch obtained documents about the fetal harvesting practices of the University of Pittsburgh, which revealed communications in which the university informed the National Institutes of Health (NIH) about efforts to “develop a pipeline to the acquisition, quality control and distribution of human genitourinary [urinary and genital organs and functions] samples obtained throughout development (6-42 weeks gestation).”77 The documents also described the university’s practice of “minimiz[ing] ischemia time” to “ensure the highest quality biological specimens.” The NIH defines ischemia as “lack of blood supply to a part of the body.”
In response to the document release, David Daleiden, whose undercover reporting brought Planned Parenthood’s disturbing abortion practices to light in 2015,78 explained that if ischemia does not start until after the “tissue collection procedure,” that means “the organs are still receiving blood supply from the fetal heartbeat during the ‘tissue collection.’”79 In other words, Daleiden continued, researchers “are allowing babies, some of the age of viability, to be delivered alive, and then killing them by cutting their kidneys out.” These and other reports attest not only to criminal abortion practices, but to the existence of a quite large fetal tissue market.
The previously mentioned Select Investigative Panel report on abortion practices in the U.S. also rebutted many of the claims made by proponents who argue for the necessity of human fetal tissue research:
“Scientific societies and universities have made misleading claims about fetal tissue research: The Select Panel has received letters from 21 institutions that claim to provide evidence for the value of human fetal tissue research. The assertions of these letters fall into 8 general classes and have been uncritically repeated in the Minority report. In reality, not a single responding institution provided substantive evidence for the value of fetal tissue research [emphasis in original].”76
The discoveries about the creation of fetal cell lines raise quite a few questions about how scientists are creating the animal cell lines that are necessary to make lab-grown meat—which is billed as being “slaughter-free,” “clean,” “guilt-free,” and “animal-friendly.”
Stem Cells
The NIH provides significant funding for stem cell research: $1.391 billion in 2014, and an estimated $1.429 billion and $1.495 billion in 2015 and 2016, respectively.80,81 There are four broad categories of stem cells: adult stem cells; fetal stem cells; embryonic stem cells; and “induced pluripotent stem cells” (iPSCs) produced since 2006 through genetic engineering (see “Stem Cell Discoveries”). To make iPSCs, scientists “reprogram” cells from adult tissue into a “stem cell-like state.”82
Stem cells have a number of properties that are appealing to researchers. First, they proliferate quickly and have the potential for self-renewal.83 In addition, certain stem cells—embryonic stem cells and iPSCs—are in an undifferentiated (“pluripotent”) state that makes them “programmable” and able to develop into any of the body’s other cell types. However, describing the state of affairs with embryonic stem cell research, MIT Technology Review lamented in 2016 that despite considerable hype, promise, and investments:
“[N]o field of biotechnology has promised more and delivered less in the way of treatments than embryonic stem cells. Only a handful of human studies has ever been carried out, without significant results.”84
Fetal stem cells are a different matter. Fetal tissue is rich in stem cells, and for this reason, scientists consider it a valuable material for research and medicine.85,86 As a 2015 news report emphasized, “What most researchers are after if they use fetal tissue are the stem cells.”87 A particularly desirable characteristic of fetal stem cells—from the standpoint of researchers not morally troubled by the harvesting techniques—is that they “grow well in lab dishes and can be kept alive and thriving for years.”
Stem Cell Discoveries
- In 1978, scientists discovered the first blood stem cells in human umbilical cord blood.
- In 1981, researchers used mouse embryo cells to create the first embryonic stem cell line.
- By 1998, scientists had derived and were culturing human embryonic stem cells.
- Fetal stem cells are isolated from fetal blood, bone marrow, or organs such as the kidney and liver as well as amniotic fluid, the placenta, and the umbilical cord—and supplied from “miscarried, stillborn, live born, or aborted human fetuses.”
- Adult tissue also contains stem cells, but the technology to use them is not yet well-developed.
- To date, stem cells derived from cord blood are the only stem cell-based products that the FDA has approved for medical use.
Sources:
“Stem cell basics.” National Institutes of Health. Accessed Jan. 24, 2023.
“Stem cell research—timeline.” Science Learning Hub, Nov. 16, 2007 (updated Aug. 16, 2018).
Lin, Sabrina and Prue Talbot. “Methods for culturing mouse and human embryonic stem cells.” Methods Mol Biol. 2011;690:31-56.
Reynolds, Matt. “The hunt for the master cow that will feed the world.” Wired, May 25, 2021.
Wallenmeyer, Petra. “The ethics of human embryonic, fetal stem cell and fetal tissue research.” Human Defense Initiative, Jul. 23, 2019.
Ultimately, what is important to understand is that fetal stem cell lines—as well as fetal tissue research and fetal tissue transplants—cannot happen without the widespread practice of abortion, which, according to the Select Investigative Panel report, typically takes place in close collaboration with research institutions.76 In a 2020 article titled “Human Fetal Tissue from Elective Abortions in Research and Medicine: Science, Ethics, and the Law,” authors from the Charlotte Lozier Institute (a pro-life think tank) documented the scale and implications of these practices:
“Since the U.S. Supreme Court issued its landmark decision in 1973 to legalize abortion, over 60 million preborn have been killed by elective abortion. While alive in the womb, these preborn are abandoned and not protected under current law. But once aborted, their body parts are a highly esteemed and prized commodity amongst certain members of the scientific community. Moral discourse is disregarded for the sake of science.”88
Tissue Engineering and Regenerative Medicine
Tissue engineering takes cell culturing a step further and tries to make cells adhere to “scaffolds.” The intent is to create a functional engineered tissue that can be adopted into the body to restore damaged tissues or organs. Because artificial tissues lack a vascular system to supply cells with nutrients and remove waste, the tissues that are currently available have a maximum width of about two hairs. In the lab, researchers have managed to create more complex organ tissues from the heart, liver, and lung, but these experimental tissues are not yet ready for actual transplants.89
The term “tissue engineering” is often used interchangeably with “regenerative medicine.” However, the first focuses more on providing living structures from cell cultures, whereas the latter more explicitly emphasizes stem cell technology.90 Both are considered promising for the field of longevity research.91
It is important to note that tissue engineering technology is identical to the production process for synthetic meat. Only the end goal—producing food or medicine—is different.
Gene Editing
Gene editing—also referred to as “new genomic techniques” (NGTs) or “new breeding techniques” (NBTs)—is applied in the development of bacterial, plant, and animal cell lines and differs from earlier rounds of genetic engineering.
- Traditional genetic engineering inserts foreign DNA into an organism, thus creating transgenic species.
- Gene editing instead uses “molecular scissors” to make targeted cuts in DNA strands; after being “edited,” the organism repairs its DNA around the molecular cut. These “small” changes to the DNA are supposed to allow for the emergence of new traits into the organism.
There are many gene-editing techniques, with CRISPR/Cas being the most well known. Other techniques include TALENs, zinc finger nuclease (ZFN) technology, oligonucleotide directed mutagenesis (ODM), cisgenesis/intragenesis, RNA-dependent DNA methylation (RdDM), and more.92
Gene editing has allowed industry to work around existing GMO legislation, with the argument being that because the changes are very small and hypothetically might occur spontaneously in nature, they should go unregulated. Unfortunately, targeted or “precise” gene-editing cuts do not equate with predictability, and so-called “off-target effects” are quite common. For example, a plant may start to produce toxins, or cows “edited” to be hornless may start to produce antibiotic-resistant genes.93
Initially, the labels “new genomic techniques” and “gene editing” were supposed to sound less harmful than genetic modification, but for the public, they still turned out to be too reminiscent of GMOs. In a campaign mounted by the Dutch consulting firm Schuttelaar and Partners—acting as lobbyists on behalf of biotech and agribusiness for the deregulation of NGTs in Europe—the firm coined the “new breeding technique” terminology to further deflect from the underlying genetic manipulation. Given that NBTs are applied expressly to avoid conventional breeding, the term is deliberately misleading. Nevertheless, the campaign was quite successful, with the NBT term becoming widely adopted in European policy circles and beyond.94
The European Commission has suggested that these next-generation GMOs are the only way it can achieve its ambitious targets to increase organic farming by 25%, decrease pesticide use by 50%, and decrease fertilizer use by 20%—all by 2030.95 Because the new GMOs are “edited” to be drought- and pest-resistant, the claim is that they will be climate-resilient and need no pesticides. Critical observers recognize this slippery line of argumentation, which is exactly the same as the one used for the earlier versions of GMOs. In reality, this playbook will leave everyone—farmers, consumers, nature, insects, and animals—worse off, except for those holding the patents to their “creations.”96-98
Precision Fermentation
Just as genomic “breeding” has little to do with traditional breeding, precision fermentation should not be confused with the fermentation methods we are familiar with, which utilize naturally occurring microbes to produce fermented foods and beverages such as sauerkraut, pickles, beer, wine, and miso.
Precision fermentation, also called “recombinant protein production,” uses genetically modified microbes (yeast, bacteria, or fungi) as “little factories” or “replicating machines.” Manufacturers insert a fragment of DNA from a plant or animal—or synthetic DNA—into a host microorganism. In large fermentation vats, these microorganisms will proliferate quickly, while producing the targeted substance.
Precision fermentation products involving animal proteins are already being commercialized as “milk,” “butter,” and “collagen,” and microorganisms can also be programmed to produce “plant-based” items such as stevia and vanilla as well as vitamins and enzymes. Outside the food industry, the process has been used for industrial chemistry, biomaterials, therapeutics and medicine, fuels, and fertilizers—the possibilities are endless!99
Synthetic Biology
Synthetic biology or “synbio,” according to the World Economic Forum (WEF), is “the engineering and redesign of biological systems that do not already exist in nature.” A synthetic biology website explains:
“Simply put, synthetic biology is an engineering approach to biology. The goal is to create and use tools that allow us to design and build functions in cells. It’s really that simple, but there’s tremendous power in that idea.”100
Synbio looks at cells as if they were little machines or “like little computers.” The same site asserts:
“[Cells] sense many factors in their environment, add those factors together, and compute the best response to their situation. Synthetic biologists can use this ability to program smart cells.”
Synbio takes a broad, multidisciplinary approach in its ambitious aim to redesign nature’s processes.101 Going beyond “traditional” gene manipulation methods, synbio researchers synthesize DNA that is designed with computer models, and create substances—and organisms—that are completely foreign to nature. After creating the first synthetic genome and transplanting it into a bacterium, geneticist Craig Venter declared: “This is the first self-replicating species we’ve had on the planet whose parent is a computer.”102
Benjamin Wolfson, a molecular scientist who wrote an overview of the history and current developments in synbio, describes synthetic biology as “one of the fastest growing fields in terms of both information and capital generation.”103 Between 2009 and 2017, he reported, private capital invested over $5.4 billion in over 160 synbio companies. In 2020, SynBioBeta, a synbio industry collaboration platform, disclosed it was tracking more than 700 synbio companies.104 In a report published in 2020, McKinsey envisioned that “60 percent of the physical inputs to the global economy could, in principle, be produced biologically,” meaning through synthetic biology. According to the consulting firm, this biotech revolution “could have a direct economic impact of up to $4 trillion a year over the next ten to 20 years.”99
In addition to venture capital, investments from the U.S. Department of Defense (DOD) are driving the synbio field—$820 million between 2008 and 2017, according to Wolfson—with two-thirds (67%) of that funding going to DOD’s DARPA branch. DARPA’s Living Foundries Program, initiated in 2012, shows how this money was not only invested in secretive projects but has spurred other actors in society to apply synbio approaches by working with academic and corporate researchers and “new players” who “will not have to be experienced in genetics to design new biological systems.”105
A related technology, molecular farming, has been described as “synbio on steroids.”106 The technique genetically modifies plants to produce targeted substances—“new compounds and products”—that may include animal DNA. The Non-GMO Project gives the example of a pea or soy crop “modified to create meat proteins, then harvested so that the end product, the pea or soy isolate, has greater protein levels for use in a plant-based burger.”
The widespread adoption of synthetic biology is not without risk (see “Synbio Wild Cards”). EcoNexus, a research organization that analyzes and reports on new technologies that could significantly impact ecosystems and biodiversity, warned over a decade ago that the synthetic organisms created through synbio come with even more unknown risks than traditional GMOs:
“At the moment the discussion of what is a sufficient or appropriate risk assessment for a GM crop is still ongoing. For other organisms such as GM trees or GM insects there are not even tentative agreements or cases to which one could refer. GM algae do not even appear to have been discussed so far. If these questions have not been solved for GMOs, then there is no basis for assessing the more far-reaching cases of Synthetic biology and extreme genetic engineering.” 105
Despite the lack of “clear regulations regarding risks and responsibility thereof,” EcoNexus cautioned in 2011, hundreds of synbio companies are now producing synthetic DNA and completely new, unnatural organisms.
One of the more notable synbio start-ups is Ginkgo Bioworks, a company that engineers custom-made microbes for the chemical, pharma, and food industries. Their value offering is a biological engineering platform. Ginkgo is planning to build up to 20,000 of these “little factories” for customers using a business model that they liken, in their pitch deck, to that of Amazon.107
Noteworthy Ginkgo investors are Bill Gates, who invests heavily in synbio “solutions,” as well as DARPA and the start-up accelerator Y Combinator. Through a merger with the SPAC called Soaring Eagle, the company went public in May 2021 at a valuation of $17.5 billion.108 In 2022, Ginkgo partnered with IARPA to create a tool that can detect biological weapons.109
Synbio Wild Cards
While the mass media and business press are fawning about the technological potential of companies like Ginkgo Bioworks, its Securities and Exchange Commission (SEC) listing (as quoted by the Organic & Non-GMO Report) suggests some caveats:
“The genetically engineered organisms and materials that we develop may have significantly altered characteristics compared to those found in the wild, and the full effects of deployment or release of our genetically engineered organisms and materials into uncontrolled environments may be unknown…. Such deployment or release … could impact the environment or the health and safety of our employees, our customers’ employees, and the consumers of our customers’ products.”
Ginkgo’s risk disclosure also admitted:
We cannot eliminate the risk of (a) accidental or intentional injury or (b) release, or contamination from these materials or wastes, which could expose us to liability.”
Source:
Ken Roseboro. “GMO 2.0 promises: We’ve heard this before.” The Organic & Non-GMO Report, Oct. 3, 2022.
Perhaps more so than with any of the other new technologies, synbio exposes the hubris of transhumanism, for it is completely blind to the risks introduced by its “sorcerers’ apprentices” and their experiments. This overreach, and the real intentions behind the mass introduction of this technology, is best illustrated by the WEF, which boasts about the coming of a “Synthetic Age”:
“In the Synthetic Age, it is not just Earth’s products that are impacted by human actions. Earth’s formative processes themselves become open for redesign. […] There are plenty of compelling reasons to think that these types of technologies could be highly desirable.”
Quoting Harvard professor George Whiteside’s enthusiastic statement that “it would be a marvelous challenge to see if we can out-design evolution,” the WEF continues:
“The Synthetic Age reconfigures the essential background substrate out of which all of human history has been crafted. It calls for replacing a world that is ‘found’ with a world that is ‘made.’ It cuts Homo sapiens loose, perhaps, from important psychological anchors located deep in the recesses of time.”102
V. How the “Sausage” Gets Made
The production of synthetic meat involves all of the techniques discussed in the previous section (see Figure 11):
- The process starts with stem cells from a live animal.
- The cells are then cultured in a medium that mimics blood and often contains real blood.
- When the stem cells have sufficiently proliferated, they are induced to differentiate into cells that are usually found in meat.1
- Finally, the cells are harvested.
- To create a structure that is reminiscent of meat, additional production steps may be applied.
Each of these steps comes with its own specific challenges, discussed below.
Challenge #1: The Base Material (Stem Cells and Cell Lines)
To create synthetic meat, cells must be able to proliferate and create adequate quantities. They must also be able to differentiate into the cell types—muscle and fat—that make up real meat. Under normal circumstances, however, cells do not proliferate, doing so only when a fetus grows or when a cancer is raging. This explains the preference for stem cells as a base material for synthetic meat cultures; they have a great capacity for self-renewal and proliferation and pluripotent stem cells can be changed into other cell types.
Lab meat manufacturers isolate their stem cells from biopsies of live cattle, either using them directly in a primary culture or using the products of biopsy to create cell lines. Both options come with disadvantages that make large-scale production challenging:
- Primary cell disadvantages: Although primary cells are considered more “animal-friendly” (because they do not require slaughter), they require a donor herd. Moreover, primary cells have a limited capacity for proliferation.110 Because primary cells require numerous biopsies, variability is also introduced into the production process. This can create problems with both safety and quality.111
- Cell line disadvantages: Immortal cell lines decrease the dependency on a donor herd and are easier to culture in a lab. David Humbird, an experienced chemical engineer who conducted a two-year feasibility assessment of lab-grown meat, asserts that cell lines will be necessary for large-scale production. He suggests using stem cell lines from adult stem cells, embryonic stem cells, or genetically manipulated stem cells.112 However, the use of stem cell lines requires assessment for possible tumor-generating characteristics, because stem cells may express genes that can cause cancer.14,110 Cell lines are also costly and time- and resource-intensive to develop, with the GFI reporting that it often takes 6 to 18 months “to derive and sufficiently characterize a single line.”113 It may be even more challenging than that: the first usable cell line from a cow was only created in 2018.114
Interestingly, access to immortal cell lines from the species whose meat we typically consume is considered the “Holy Grail” of the synthetic meat sector. “Cell lines are the secret sauce of the cultured meat industry,” wrote Matt Reynolds in an article for Wired magazine about the “secretive world of cultured meat,” arguing that the limited availability of such cell lines is holding the whole sector back.114 Synthetic meat researchers Emily Soice and Jeremiah Johnston agree, describing the “need to produce immortal, food-relevant cell lines” as “one of the most pressing challenges of cellular agriculture.”14 In their 2021 scientific review titled “Immortalizing Cells for Human Consumption,” they described the cultured meat conundrum, namely that the “cell types and species that consumers may accept most readily for consumption differ from the type and species used in other cell line applications.” The closest existing cell lines, they commented, are from “model species commonly used in research such as mice, rat, hamsters, and Japanese quail.” In other words, the two authors emphasized, consumers are part of the equation:
“Although work in immortal cell lines began more than 50 years ago, there are few existing cell lines made of species and cell types appropriate for cultured meat. Cells in cultured meat will be eaten by consumers; therefore, cultured meat cell lines will also require unique attributes not selected for in other cell line applications.”
Observing that “Currently … there are no cultured-meat-appropriate cell lines available to researchers and developers,” the two authors concluded:
“Although there are several techniques used to establish cell lines appropriate for biological or medical research, there are still several unanswered questions about how to establish immortalized cell lines suitable for human consumption as cultured meat….” 14
Even the GFI admits: “[I]f cultivated meat is to be available for every commonly-eaten animal species, there exists significant work in the establishment of bonafide embryonic stem cell lines from a diverse set of species.”113 As the published literature shows, protocols for use of pluripotent stem cells deal mostly with humans and mice—and while scientists have ample experience using human cells to create iPSCs, that is not the case with livestock species.111
Referring to the lack of access to and information about cow cells, synthetic meat researcher Andrew Stout from Tufts University has explained, “We’re relying on a mouse cell because it’s an available mammalian muscle cell line. Really, it’s the only one.”114 Even more concerning than the use of mouse cell lines in lieu of cow cell lines are the remarks of the authors of an article focused on challenges in bringing cultured meat to market; they acknowledge that while “human primary cell sources” are available for research, “for this particular source, the culture of human tissue for meat would have enormous ethical, health, and regulatory implications.”115
Recognizing that availability of cell lines is a bottleneck challenge for the synthetic meat sector, GFI has funded multiple research projects for cell line development. In addition, in 2021 it partnered with pharma reagent provider Kerafast to establish a publicly accessible cell bank.116 So far, however, only one cell line has been deposited.14
Challenge #2: The Growth Environment (Cell Culture Media)
Cells in a living organism are sustained by blood. To keep cells alive and make them proliferate, they need an environment that mimics blood. These so-called “cell culture media” are complex, expensive mixtures that include,117 among other things:
- Amino acids (to build proteins)
- Vitamins (necessary for cellular growth)
- Carbohydrates (for energy)
- Inorganic salts
- Basic and trace elements (for example, iron, potassium, magnesium, and zinc)
- Hormones (for growth and proliferation)
- Antibiotics
Regular blood contains these substances naturally, which is why cell culture media are often supplied with fetal bovine serum (FBS)—fetal calf blood—for successful growth of mammalian cell lines.111,112 In 2021, the global market for FBS was valued at $879.9 million and predicted to reach $1.4 billion by 2030, with North America expected to lead demand for the product.118 However, FBS—tapped from living cow fetuses—challenges the “clean” and “slaughter-free” premises of lab-grown meat. In the article “The Gruesome Truth About Lab-Grown Meat,” Slate author Nick Thieme gives a graphic description of how this product is derived:
“FBS, as the name implies, is a byproduct made from the blood of cow fetuses. If a cow coming for slaughter happens to be pregnant, the cow is slaughtered and bled, and then the fetus is removed from its mother and brought into a blood collection room. The fetus, which remains alive during the following process to ensure blood quality, has a needle inserted into its heart. Its blood is then drained until the fetus dies, a death that usually takes about five minutes. This blood is then refined, and the resulting extract is FBS. Millions of fetuses are slaughtered this way.”119
Most other articles that deal with the development of cell lines from cows do not detail the process by which the cell lines are derived. It all starts with the biopsy—which amounts to taking a small piece of meat from a live animal. The researchers who have investigated the creation of human fetal cell lines have uncovered the fact that though the process is not painless, scientists withhold anesthesia to conserve cell quality. Although one article notes that calf biopsies “often occur under local anesthesia,”120 are we to believe that cows are being treated more humanely than human babies?
Just as with primary cells, the use of FBS also introduces variability into the production process—something that one would wish to avoid if scaling up a very sensitive process. Even more concerningly, its use introduces the risk of prion contamination.112,121 Prions are connected to “mad cow disease” and Creutzfeldt-Jakob disease in humans.
Manufacturers are actively seeking alternatives to FBS, with most produced using genetic manipulation or precision fermentation. For example, the synthetic meat company UPSIDE Foods is genetically manipulating cells to produce its own serum,114 while Tiamat Sciences—a biotech supplier to the synthetic meat sector—is genetically manipulating plants that will produce the ingredients for culture media.122 Various precision fermentation companies are developing culture media using GMO bacteria.18 However, both regular blood and FBS contain hormones and growth factors (proteins that make cells grow), and while it is possible to add synthetic alternatives to the medium, this will create a regulatory hurdle in the EU, which prohibits adding hormones and growth factors to foods.123
With or without FBS, culture media remain prohibitively expensive and contribute to a large extent to the high cost of producing synthetic meat. For example, FBS can cost over $1000 per liter.111 Essential 8, an FBS-free culture medium, costs $376.80 per liter.121 Other media can increase the cost of synthetic meat to over $20,000 per kilogram.64,124 GFI is optimistic about possibilities to bring medium costs down, whereas chemical engineer David Humbird is more reticent; according to his assessment, even at economies of scale, the ingredients remain costly and at times difficult to source.
Challenge #3: Nanotech Structures for Fake Meat (Scaffolding)
Because synthetic meat is supposed to look and feel like real meat, the slurry of cells that is the end product of the culturing process needs a structure or scaffold. Real tissue is made up of fibers, blood vessels, and connective tissue—and contains a variety of cells, such as muscle and fat cells—whereas cell cultures not only lack structure but also an immune and capillary system to provide the cells with oxygen and remove waste products.123 Because of this, cell cultures can only grow in layers of about 0.2 to 0.5 millimeters thick.59,125 This is why current synthetic meat products are often chicken nuggets or ground meat.123 The lab-grown burger that made its debut in 2013, consisting of 70% synthetic “meat,” was supplemented with breadcrumbs, egg powder, and spices to add taste and structure.
State-of-the-art tissue engineering has developed five nanotechnology-reliant techniques to create more elaborate structures (see “Nanotech: Five Tissue Engineering Techniques”).126-130 The U.S. National Nanotechnology Initiative defines nanotechnology as nanoscale science, engineering, and technology that involves “imaging, measuring, modeling, and manipulating matter between approximately 1–100 nanometers”;131 a nanometer is one-billionth of a meter and one-millionth of a millimeter.
In the Solari Report titled “Medical Nanobots: Implications of the Wave Genome,” researcher Ulrike Granögger explains the implications of the growing use of nanotechnology:
“This nanoscale of reality that we are entering with new technologies—especially medical technologies—is more than simply a tiny, miniature form of material engineering. It is, in fact, a phase transition. It is a literal threshold between matter and energy, between the particulate and the waveform that we are entering as we go to the domain of sizes and of objects on the micro- and nanoscale reality.”132
For the purposes of synthetic meat, tissue engineering scaffolding technologies need to be edible. With the scaffolds that are currently available, this is not always the case.
Nanotech: Five Tissue Engineering Techniques
- Microbeads: Microcarriers are little beads that can be added to the culture to provide a surface to which the cells can adhere. This helps to increase the production volume. If the beads are not edible, they need to be removed at the end of the production process, which is challenging and lowers the yield.
- Porous scaffolding: Porous scaffolds (as well as fiber scaffolds) create a nanostructure to which the cells can attach, and through which the medium can circulate. The porous structure is spongelike.
- Nanofiber scaffolding: Fiber scaffolds feature long-spun nanofibers. The CEO of Matrix Meats—which makes nanofibers for synthetic meat—has stated that the company’s products are edible, but the scaffold’s online patent includes a very long list of chemical-looking polymers, including nylon. According to the patent, “electrospun polymer fiber comprises a polymer configured to degrade upon exposure to saliva.” In other words, this scaffolding contains plastics that “degrade” in your mouth.
- Hydrogel nanoparticles: Hydrogels are polymer structures that can absorb a lot of water but do not dissolve in water. They can be “tuned” to respond to environmental triggers and to incorporate other molecules. Because they mimic soft tissue, they are widely applied in tissue engineering.
- 3D printing: With 3D printing, nanoengineered bioinks that contain cells are printed layer by layer. The bioinks used in synthetic meat are usually hydrogels.
Challenge #4: Bioreactors
Cell cultures begin in a flask, moving on first to a small vat and then, when the culture proliferates further, to a larger vat called a bioreactor. The size of the bioreactor needs to fit the culture; otherwise the cells will not grow. A single facility will need hundreds of bioreactors (see “A Hypothetical Manufacturing Plant for Lab-Grown Meat”).64
The bioreactors currently in use in the biopharma industry are maximally 20,000 liters.133 However, scaling up production of synthetic meat will require even larger reactors and significant innovations.126 Eat Just CEO Josh Tetrick suggests that his company will need bioreactors with a 100,000-liter capacity to meet the goal of producing 10 million pounds of synthetic meat per year.
Industry experts and chemical engineers interviewed by food journalist and editor Joe Fassler contest the feasibility of reactors of the size envisioned by Tetrick.64 In an extensive article published in 2021, titled “Lab-grown meat is supposed to be inevitable. The science tells a different story,” Fassler investigated the viability of lab-grown meat more generally, presenting findings discussed in greater detail below.
The experts interviewed by Fassler indicated that as the size of vats increases, quality control needs to be near perfect, and outlined a series of problems with larger batch sizes:
- They are more easily contaminated. David Humbird, cited earlier, explained that a 100,000-liter bioreactor “has never been done because you can’t. You’re just going to be producing vats of contaminated meat over and over again.”
- The smallest contamination can ruin a whole batch, which can be very costly.
- As cells grow, they produce toxins, and because there is no capillary system to discard the toxins, they accumulate in the culture. Again, increasing the batch size increases this problem. Although intermediate steps can be taken to clean up the culture, these require extra bioreactors.
- Large vessel sizes can place too much stress on the cells, causing them to die off and “turn into this kind of slimy stuff that’s really horrible,” according to former Pfizer scientist and biotech consultant Huw Hughes, who stated, “You really can’t afford to have that happen.”
A Hypothetical Manufacturing Plant for Lab-Grown Meat
In a technological assessment published by the Good Food Institute in March 2021, GFI ran the numbers for a hypothetical synthetic meat plant. A single plant would:
- Come with a price tag of $450 million
- Need more than 130 production lines and more than 600 bioreactors (roughly amounting to one-third of the world’s current biopharma bioreactor volume)
- Produce 22 million pounds of lab meat per year, representing 0.022% of annual meat production in the U.S.
To increase synthetic meat production to 10% of the world’s meat supply by 2030, it would be necessary to build 4,000 of these hypothetical plants—at a minimum cost of $1.8 trillion. One expert interviewed by journalist Joe Fassler ran a fact-check on GFI’s numbers and concluded:
“GFI’s report projected unrealistic cost decreases, and left key aspects of the production process undefined, while significantly underestimating the expense and complexity of constructing a suitable facility.”
The most optimistic scenario would bring the price of synthetic meat down to $17 per pound, but not for the end product—for a cell slurry containing 30% cells and 70% water. Taking into account cell biology, input costs, economies of scale, and factory design, another of Fassler’s experts asserted: “The cost of cultivation facilities will always be too burdensome, and the cost of growth media will always be too high, for the economics of cultured meat to make sense.” He also noted that it was “hard to find an angle that wasn’t a ludicrous dead end.”
Source:
Joe Fassler. “Lab-grown meat is supposed to be inevitable. The science tells a different story.” The Counter, Sept. 22, 2021.
Challenge #5: Quality Control
To keep products free of contamination, biopharma manufacturers employ pharma-grade management systems that are certified as Good Manufacturing Practice (GMP). The standards for food production require much less stringent quality controls. GFI appears to be making the assumption that synthetic meat producers will follow food standards, but—whatever the regulatory requirements are going to be—the nature of producing cell cultures will require pharmaceutical production standards.
In his feasibility assessment, Humbird outlined the necessity of cleanrooms—areas that meet GMP requirements for tight control of airborne particles. Depending on the cleanroom grade, employees might need to decontaminate and put on hazmat suits before entering the production area. Cleanrooms add significantly to operating costs and energy bills.
In his interview with Fassler, Humbird stated:
“You can make a big plant, or you can make a clean plant. So if you want to feed millions and millions of people, it’s got to be big. But if you want to do it with animal cells, it’s got to be clean. We need both, and you can’t do that.”
Challenge #6: Antibiotic Resistance
Antibiotic resistance is widely recognized as a mounting problem, with the overuse of antibiotics in industrial farming seen as a large contributor. An often-repeated but false argument in favor of synthetic meat is that because this “meat” is produced in a sterile environment, it will not require antibiotics—this is not true.
Multiple sources who understand cell culture production processes acknowledge that use of antibiotics and fungicides is not only very common but necessary if one wishes to avoid the contamination that spoils product batches.123 Bill Freese, science director at the Center for Food Safety, has commented:
“Antibiotics and anti-fungal drugs will be unavoidable if cell-cultured meat facilities scale up to any size, and this can only worsen the resistance epidemic that is rendering these life-saving medications useless for ever more people.”134
Challenge #7: Pricing
As already mentioned, Singapore is currently the only place in the world where you can legally buy a piece of lab-grown meat. The Singapore Food Agency approved the sale of Eat Just’s lab-grown chicken nuggets in December 2020.135 Even at $17 a dish, Eat Just is selling the nuggets at a loss. The company is not willing to specify the real production costs, but in a 2021 email to Mother Jones, Eat Just’s head of global communications glowingly wrote: “Our cultured chicken costs continue to drop with more than a 90 percent decrease in media and total costs since 2018. We have a clear path to continue reducing our costs below that of conventional chicken, which will happen in the coming years ahead as we expand our manufacturing capacity and continue to improve our process.”26
Regulators have allowed some companies to sell their products during tasting events, even in the absence of regulatory approval. At one such event in Israel, Supermeat sold its chicken burger for $35.136 Fassler reports that the lab-grown fish company Wildtype, based in San Francisco, is “currently trying to get its costs for cell-cultured fish down from hundreds of dollars a pound to five or six dollars a pound” but does not provide details on how the company plans to achieve this. Future Meat Technologies claims it is producing lab-grown chicken for $18 per pound, but this grossly misleading calculation only includes input and utility costs and excludes all other expenses.64
GFI also applies optimistic—and creative—cost calculations. It proposes that the cost of synthetic meat can be lowered by assuming a repayment term of 30 years. GFI’s CEO Bruce Friedrich suggests that if the private sector is not forthcoming with lenient investment terms, the public sector should invest billions to bring lab meat to market.64
Challenge #8: Environmental Impacts and Waste
“Cultivated meat can massively reduce the climate impact of meat production,” writes GFI. They cite a study by Dutch research firm CE Delft, which claims that compared with conventional beef, “meat cultivated directly from cells may cause up to 92% less global warming and 93% less air pollution and use up to 95% less land and 78% less water.”137
How realistic are these claims? As we have seen, the cell-culturing process is complex, challenging, and resource-, capital-, and knowledge-intensive.26,112 Because no data for a real operating plant are available, most projections about the environmental impacts of still-hypothetical plants are guesswork.138 A fair environmental impact assessment would need to take into account, at a minimum:
- The energy required to operate hundreds of bioreactors and cleanrooms in 4,000 production plants
- The resources required to build these plants, including the impact on natural systems
- The cleaning and disinfection agents needed to keep the plants free of contamination, and the impact on water usage
Writing in 2018 about lab-grown meat technical and regulatory challenges, a group of UK authors commented that “waste removal strategies require attention.”115 In the biotech and pharmaceutical industries, waste products from production processes and lab activities may be classified as hazardous waste, depending on the jurisdiction. Both the waste water and waste products can be contaminated. Those circumstances require processing by a specialized waste management entity.
However, a 2021 literature review of published environmental assessments of cultured meat production systems revealed a notable absence of research, data, or information about waste management.139 Assuming that the same waste management regulations apply to cultured meat production as to pharmaceutical manufacturing, this would not only increase costs but add to environmental impacts. The UK authors speculate that cultured meat waste products might come to be considered “animal by-products” and that “facilities of different scales [might] require different regulatory pathways.”115
VI. Safety and Nutrition: Afterthoughts?
Safety
Not surprisingly, perhaps, little to no information is available about the health implications of eating biopharma cell constructions comprising cell lines, GMOs, nanotech, and fetal bovine serum. In a review focusing on the “Safety of Alternative Proteins,” authors Joshua Hadi and Gale Brightwell ascertained that “most studies focus[] on technological improvements for better production means” rather than studying the safety of cultured meat.140
From consumers’ standpoint, however, “one of the most common concerns about cultured meat … is the safety of ingesting cell lines”; as the authors of the article “Immortalizing Cells for Human Consumption” point out, “Currently, cultured animal cell lines are not commonly eaten by consumers.” The public’s concerns may be justified, as it is not yet confirmed if these cells can cause cancer. As the authors add:
“[D]ue diligence would require further investigation in genetically engineered animal cells. Confirming that future products made from immortalized animal cells expressing oncogenes, either through spontaneous immortalization or genetic engineering, would be safe represents a gap in knowledge in this field.”14
New Harvest jumped in to fill this research gap by crowd-sourcing self-assessments of its industry members in a series of workshops.141 Although the resulting paper “wasn’t academic in the classic sense,” it passed peer review. Applying existing safety assessments of food and pharma processes, participants made the assumption that “most hazards are not expected to be novel.”120 Nonetheless, the list of risks that industry representatives identified was impressive (see “Potential Manufacturing Hazards of Cell-Cultured Meat and Seafood”).
Potential Manufacturing Hazards of Cell-Cultured Meat and Seafood
When 50 leading companies “shared previously unpublished details about their manufacturing process … to inform safety recommendations for cell-cultured meat and seafood” and published their findings in a peer-reviewed journal, they came up with the following list of potential manufacturing hazards that could result in a product unsafe for human consumption:
- Microbiological contamination “with bacteria (including mycoplasma), fungi, and viruses,” which, they acknowledged, “can occur throughout the entire manufacturing process”
- Potential increases in antibiotic resistance
- Allergens and growth factors in the medium that “could be hazardous to human health”
- Presence in the final product of chemicals to protect cells (cryoprotectants) “in amounts not safe for human consumption”
- Cell culture contamination with prions
- Introduction of “infectious disease agents” into cell cultures, changing product characteristics
- Unplanned gene expressions and “genetic drift,” potentially causing unexpected health effects
- Hazardous substances in the scaffolds and microcarriers
- Hazardous processing agents
- A theoretical possibility that genetically modified DNA in the “meat” could “integrate or be transferred” to the gut microbes
Nevertheless, the industry-affiliated authors remained optimistic: “Cell-cultured meat and seafoods that are biochemically, genetically, and compositionally similar to existing foods should theoretically be as safe as their conventional counterparts.”
Source:
Kimberly J. Ong et al. “Food safety considerations and research priorities for the cultured meat and seafood industry.” Compr Rev Food Sci Food Saf. 2021;20(6):5421-5448.
Consumer protection agencies have a different perspective and are issuing warnings about the risks of eating lab-cultured meat products. Jaydee Hanson, Policy Director of the Center for Food Safety, cautioned in 2020:
“Of particular concern is the genetic engineering of cells and their potential cancer-promoting properties. To be able to better assess whether the products are being produced by methods that involve genetic engineering and use genetic constructs … that might encourage cancer cells, we need more information on how the cells are engineered and kept growing. Many of the companies are claiming this information is confidential and a business secret.”
The food safety advocate soberly added:
“The companies have also not disclosed plans for how they will dispose of the toxins from bioreactors, scaffolding, and culture media like growth factors/hormones, differentiation factors, often including fetal calf serum or horse serum, and antimicrobials…. If companies can’t find a way for this ‘meat’ to dispose of these toxins, the long-term cultures could potentially build up within the ‘meat’ itself.”110
The hardly reassuring solution put forth by industry and New Harvest is post-marketing monitoring—observing consumers after they eat their products—to “help detect rare and unintended adverse effects such as allergic responses.”120
Nutrition
Despite claims that “synthetic meat is exactly the same as real meat,” evaluations of lab-grown meat have ignored nutritional profiles as well as safety. Real meat is nutrient-dense and supplies necessary vitamins, minerals, and other important nutrients. With synthetic meat, “the nutritional composition is unclear,” write the authors of “The Myth of Cultured Meat,” as “no strategy has been developed to endow cultured meat with certain micronutrients specific to animal products (such as vitamin B12 and iron).”123
Studies that compare the nutritional profile of plant-based burgers versus real meat have been performed. Citing a study published in Nature, Children’s Health Defense reported in 2021 that “despite having similar Nutrition Facts labels,” the nutrients differed by 90%,” meaning that grass-fed meat and plant-based meat are not “nutritionally interchangeable.”142 Even worse, researchers at Sweden’s Chalmers University of Technology in Gothenburg found that the nutritional content of most meat substitutes, such as those containing soy and beans, comes in a form the body cannot absorb.143
In a study commissioned by Impossible Foods, which makes plant-based burgers from genetically manipulated soy, rats fed the Impossible Burger got sick. The burgers include “soy leghemoglobin” (heme) to give them a “meaty taste” and appearance. After 28 days on an “Impossible diet,” the rats experienced:
- “unexplained transient decrease in body weight gain
- increase in food consumption without weight gain
- changes in blood chemistry
- decreased reticulocyte (immature red blood cell) count….
- decreased blood clotting ability
- decreased blood levels of alkaline phosphatase (can indicate malnutrition and/or celiac disease)
- increased blood albumin (can indicate acute infection or damage to tissues) and potassium values (can indicate kidney disease)
- decreased blood glucose (low blood sugar) and chloride (can indicate kidney problems)
- increased blood globulin values (common in inflammatory disease and cancer).”144
In addition, the researchers observed reproductive cycle disruptions. Despite these concerning outcomes, Impossible Foods listed them as “non-adverse,” “transient,” and having “no toxicological relevance”—and the FDA approved the soy leghemoglobin ingredient as GRAS (Generally Recognized as Safe).
VII. Global Agreements
A Cascade of Policies
If one takes a big-picture view, it is apparent that a cascade of policies and international agreements is enabling the centralization of food production and ownership—like it or not. Here is policy-makers’ ostensible logic:
- First, at the top of the pyramid, climate agreements set goals reduce greenhouse gas emissions.
- Second, at the next level down, policy-makers are targeting agriculture, stating that it is emitting too many greenhouse gases. For example, the Intergovernmental Panel on Climate Change (IPCC) warns that “even if fossil fuels were eliminated overnight, emissions from the food system alone would jeopardize the Paris Agreement target of keeping global temperature rises below 1.5C.”145
- Third, policy-makers portray food production as a dangerous activity that is threatening both human health and the environment in myriad ways. To combat these ills, reports from the United Nations, the Food and Agriculture Organization (FAO), various NGOs, the EU, and national governments are uniting in their calls for a “radical transformation” of food systems. This supposedly essential transition will require a change in people’s diets—meaning eating less meat and fewer animal products.
Meanwhile, there is an ongoing and multipronged attack on the production of real food.146-148 To help put a highly synthetic, centralized, inaccessible, and unnatural food system in place, these attacks feature:
- Patenting of seeds
- Fires at food processing plants
- Goals to “rewild nature” and leave productive land fallow
- Introduction of wolves into densely populated areas
- The blocking of fertilizer deliveries and other supply chain disruptions
- The buying up of land
- Policies that push farmers off the land, as is happening in the Netherlands
- Promotion of a neofeudal agricultural system driven by debt slavery
In short, the concerted attacks on food production—all in the name of protecting human health and the environment—are a thinly veiled power grab that seeks to decrease food independence and further consolidate centralized control over all means of food production.
Investigative journalist Corey Lynn and others have extensively described and tracked the system that is envisioned for us. It involves a combination of FoodTech technologies such as synthetic meat,149 insects for humans,150 indoor factory farms that grow GMO produce,151 a fully traced and surveilled supply chain, and even the prescribing of certain foods as medicine or “precision nutrition.”152 All of these “innovations” are leading to a system where food production no longer occurs out in the open, in fields, but instead takes place behind factory doors. Moreover, the “novel” foods are all controlled by patents—patents that are owned by a handful of corporations that already dominate almost all food supply chains.51
What follows is an overview of the major policies and coalitions that are driving the transformation of our food systems.
From Climate to Agriculture to Diet
The IPCC’s latest climate assessment (September 2022) hails plant-based and lab-grown meat as “transformative solutions.”145 The IPCC informs climate conventions such as the UN Climate Change Conferences (COP27 in 2022) and intergovernmental forums such the G20, and also trickles down to lower levels of government, creating a favorable environment for government and business funding of proposed climate solutions.153 For example, during the November 2022 COP27 meeting, 42 governments agreed to invest $8 billion into the Agriculture Innovation Mission (AIM) for Climate, an initiative to accelerate “climate-smart” agriculture solutions and “food system innovations.”149
The COP27 meeting also launched the Food and Agriculture for Sustainable Transformation (FAST) Initiative, designed to be a catalyst for food system transformation by operating as a public-private cooperation platform, enabling access to finance, and providing knowledge and policy support.154 Forbes reports that FAST will encourage a “shift towards sustainable, climate-resilient, healthy diets” and “help reduce health and climate change costs by up to US$ 1.3 trillion while supporting food security in the face of climate change.”155 After COP27, geopolitical analyst F. William Engdahl astutely observed that the real purpose behind the $1.3 trillion shift is a change in governance of global resources and the creation of a new global economic system.153 As a reminder, Engdahl quoted the famous 1974 Club of Rome report that declared:
“Nations cannot be interdependent without each of them giving up some of, or at least acknowledging limits to, its own independence. Now is the time to draw up a master plan for organic sustainable growth and world development based on global allocation of all finite resources and a new global economic system.”
As both AIM and FAST reveal, globalists’ climate “solutions” increasingly focus not just on shifting agriculture but on changing people’s diets. Engdahl noted: “At COP27, discussion was about ‘diets that can remain within planetary boundaries, including lowering meat consumption, developing alternatives, and spurring the shift towards more native plants, crops and grains (thus reducing the current reliance on wheat, maize, rice, potatoes).’”
Engdahl’s conclusion: “The war on animal raising for meat is just getting deadly serious.”153
Parallel Governance Structures
The WEF hosts a range of platforms and initiatives to advance the agenda to transform agriculture and diets. The “Future of Protein Action Community,” for example, “aims to accelerate the transformation of the protein ecosystem,” while the “Protein Action Group” works to “amplify and disseminate protein narratives and action.”156
The Netherlands—where the WEF’s taxpayer-funded Global Coordinating Secretariat (GCS) was founded (see Figure 12)—is at the center of many of these developments, with the WEF and Dutch cabinet members and high-level civil servants all working in close collaboration.157 Playing a coordinating role, the GCS connects regions with the global agenda, while the regional offices connect scientists with farmers and business to “future-proof food systems.”158 The world’s leading expert on technocracy, Patrick Wood, calls this process “devolution,” referring to the creation of new and unaccountable governance layers that bypass existing democratic structures.159 It may be informative to research which regional initiatives have been set up in your country or state.
Figure 12. The Netherlands and the World Economic Forum

The tendency to replace official governmental frameworks with unofficial parallel structures that are dominated by corporate and NGO interests has also been criticized by Sofia Monsalve, Secretary General of the human rights organization, FIAN International, which advocates for the right to adequate food and nutrition. In an interview with the Dutch paper De Andere Krant about the “corporate capture” of the 2021 UN Food Systems Summit (UNFSS), she explained the concerns:
“[The] UN Secretary General`s summary and statement of action [after the UNFSS] clarifies the intense follow-up process, especially a coordination hub led by Rome Based Agencies. It is important to note that there has been no intergovernmental decision on [these] follow-up mechanisms and processes, although this represents a major change to the global food governance. The UNSG [UN secretary-general] is therefore clearly acting outside his mandate, as do the Rome Based Agencies in supporting the proposals. This announcement is also contrary to the repeated commitment to not creating new structures—in particular by the Deputy Secretary General (DSG).
We are very concerned that the summit with its follow-up mechanisms strongly promotes a ‘multi-stakeholder’ governance model at all levels, which is opposed to multilateralism and human rights. In multilateralism governments (duty bearers) make decisions on global issues on behalf of their citizens (rights holders) which translate into obligations and commitments that states and international organizations must fulfill. In a human rights approach those most affected are at the center of decision-making and duty bearers are accountable to rights holders. In the multi-stakeholder model governments relinquish their responsibilities and power to the private sector and give them preferential access to public decision-making. This will further marginalize countries from the Global South in international decision-making and prevents the adoption of binding regulations for transnational corporations.”
Monsalve also commented, “[T]he UNFSS was not an intergovernmental meeting where specific decisions on policies were taking place, but an event convened by the UN Secretary General.” The summit led to the creation of various “action coalitions,” about which she noted, “These coalitions of action have emerged in an opaque way and are multi-stakeholder coalitions with the same governance problems as detailed above. Which concrete actions will emerge from these coalitions is yet to be seen but we are very critical as there is a heavy influence by corporations and philanthropic foundations” (see “The Philanthropists”).
The Philanthropists
A “Federal Climate Policy Playbook” produced by the billionaire think tank Breakthrough Energy—founded by Bill Gates with investors such as Michael Bloomberg, Richard Branson, and Jack Ma—illustrates the kinds of policies these philanthropists are interested in promoting. (The Gates-founded think tank should not be confused with the breakthrough energy movement [BEM], which focuses on raising awareness about the existence of real breakthrough energy technologies, such as zero-point energy and anti-gravity.) At the playbook’s release, the GFI declared it was “thrilled to contribute to [this] critical climate policy advocacy.”
According to the billionaires’ analysis, whose priority is to “decarbonize the economy,” their goal will be impossible unless people stop eating meat. To make use of alternative proteins’ “transformative potential for reducing agricultural emissions,” their playbook lists recommendations to stimulate alt-protein development. To adhere to the playbook, the federal government should:
- Increase investments into alt-proteins
- Provide tax credits and other fiscal incentives
- Adjust government procurement policies for cafeterias and official buildings in favor of offering more plant-based foods
- Develop a “rational regulatory scheme” and “sensible labeling standards”
Source:
Elizabeth Derbes. “Breakthrough Energy policy playbook highlights alt proteins’ critical role in reaching net-zero emissions.” Good Food Institute, Mar. 5, 2021.
Deregulating GMOs in the EU—Through Climate
In December 2019, the European Commission announced a “Green Deal” that commits EU member-states to becoming carbon-neutral by 2050 and reducing carbon emissions by at least 55% by 2030. Robust funds have been allocated to stimulate this transition; the EU Horizon Fund has a budget of €95.5 billion.160
Part of the Green Deal is a “Farm to Fork” strategy for a “fair, healthy and environmentally friendly food system.”161 This sub-policy sets agricultural goals to cut pesticide use by 50% and fertilizers by 20%, as well as increase organic farming by 25%. That sounds wonderful until you understand the caveat—the Commission is planning to deregulate new GMOs, deeming deregulation the only way to achieve the ambitious “Farm to Fork” goals.96 The University of Wageningen is helping advocate for allowing the new GMOs to enter the food supply labeled as organic.162
In another agreement, the biodiversity strategy, the EU’s stated aim is to “restore nature” by converting 10% of productive agricultural land into new wilderness areas.163
United States: FoodTech Takeover Via Executive Order
In the U.S., the government is doing its part to make the agricultural sector more “climate-resilient.” Strategies include USDA investments ($2.8 billion) in 70 projects for “climate-smart” agricultural solutions164 and $8 billion from the White House toward hunger and nutrition.152 On September 12, 2022, the Biden administration signaled that the U.S. has found a new angle to accelerate the shift into synthetic foods, issuing an Executive Order (EO) on “Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy.” The EO requires the Secretary of Agriculture to:
“[S]ubmit a report assessing how to use biotechnology and biomanufacturing for food and agriculture innovation, including by improving sustainability and land conservation; increasing food quality and nutrition; increasing and protecting agricultural yields; protecting against plant and animal pests and diseases; and cultivating alternative food sources.” 165
In short, the EO enables the Secretary to set loose the whole “transhumanist toolbox” on U.S. agricultural production. The order also gives executive support for McKinsey’s $4 trillion “bio revolution”99 and the WEF’s predicted dawn of a “synthetic age.”
The Non-GMO Project’s critical response to the EO cogently outlined the threats to food independence and the natural world:
“The executive order fundamentally misrepresents the nature of biological systems. It imagines genetic material and cells as a collection of programmable entities, like computers or software. This simplification of complex relationships distorts our relationship with the natural world. Building a food system based on biotechnology preserves our current extractive view of natural resources, continuing to degrade soil health, biodiversity and our ability to nourish ourselves.
With its unqualified support for novel biotechnology, the executive order will encourage the ongoing privatization of the food supply, placing even more of our shared resources into the hands of a few multinational corporations. It ensures continued prosperity for agrichemical and biotechnology companies at the expense of food security, environmental resilience, health and equality.”166
United Nations: “One Policy to Bind Them”
“One Health” is a UN policy perspective that claims that every area of nature and life—livestock farming, agriculture, food production, and us—poses a potential “biosecurity risk.” According to the UN:
“One Health involves the public health, veterinary, public health [sic] and environmental sectors. The One Health approach is particularly relevant for food and water safety, nutrition, the control of zoonoses (diseases that can spread between animals and humans, such as flu, rabies and Rift Valley fever), pollution management, and combatting antimicrobial resistance….” 167
Calling for close collaboration between all sectors of society from which biosecurity risks can emerge, the World Health Organization (WHO), UN Environment Programme (UNEP), and FAO have joined forces, putting in place a veritable infrastructure at the supranational, national, local, professional, and research levels.6
Hypothetically, should the WHO declare a zoonotic pandemic of international concern, the One Health infrastructure would be able to set into motion not only population lockdowns but measures affecting food supply chains and natural areas as well. The fact that synthetic meat is consistently put forward as a way to combat zoonotic diseases and antimicrobial resistance should be viewed as a red flag.
VIII. Behavioral Nudges
The Netherlands’ Social Engineering Experiments
In 2018, the European Commission required each EU member-state to develop a national protein strategy to support the “protein transition.” The Netherlands published its National Protein Strategy in December of 2020.
The Dutch strategy includes directions for farmers, but more interestingly, it stipulates how the government and businesses that are part of the food supply chain should collaborate to manipulate people into changing their diets. With the Ministry of Agriculture dictating the behavioral goals, the strategy proposes that a coalition of supply chain partners work to achieve them by “adapting the food environment” through options such as offering different products, altering placement of foods in supermarkets, “naming and shaming,” and even implementing a meat tax “because it gives a strong signal about the desired societal direction.” (Despite the current “woke” government in the Netherlands, this last suggestion has met with strong popular resistance and is, for now, off the table.) The jargon that the government employs conveys a “behavioral approach”—characterized by the advanced “nudging” and social engineering practices that many Western governments have now adopted as standard operating procedure—and for which Covid provided the ultimate testing ground.168
The Dutch business community has a history of industry self-regulation through covenants, so it was quick to launch Transitiecoalitie Voedsel (“Food Transition Coalition”), a coalition of “Dutch frontrunners in the world of agriculture, food, nature and health” that reportedly provides “a stage for pioneers, brings market players together and develops new business models in cooperation with ‘system players’ such as governments, the financial sector, umbrella organizations and regulators” (see “‘System Players’ Around the World”). During an October 2022 stakeholder dinner at which all the “system players” were represented—under the banner, “Plant-based: the New Normal”—European Commission Vice President Frans Timmermans emphasized:
“The problem is that Europeans live in a ‘food environment’ that does not encourage or facilitate healthy and sustainable eating. We [the European Commission] have to help citizens make an informed choice. And make the healthy choice the easy choice.”169
Taking the European Commission’s advice to heart, the Dutch city of Haarlem has decided to help lead consumers to the “easy” choice: the city has become the first in the world to ban meat advertising in public places. In the bargain, it also banned advertising for other “carbon-generating” activities like holiday flights.170
“System Players” Around the World
In 2019, the EAT-Lancet Commission published the “Planetary Health Diet,” an “independent” report intended to back up, with “scientific” recommendations, the push to change people’s diets in accordance with the UN’s Sustainable Development Goals (SDGs). The “Planetary Diet” recommendations include restrictions on the consumption of meat, dairy, and potatoes. Funding came entirely through the “generous support” of the Wellcome Trust, “which had no role in the writing of the report.” The Wellcome Trust has its roots in the eugenics movement and is pursuing its current goals through various transhumanist, population control, and vaccination projects.
EAT is a nonprofit that “connects partners across science, policy, business and civil society,” collaborating with almost 40 governments and partners such as the Berkeley Food Institute, the Consortium of International Agricultural Research Centers (CGIAR), Danone, Google food services, the Harvard Global Equity Initiative, Kearney, MIT, Nestlé, Nordic Choice hotels, and University College London. In addition, the organization works closely with alt-protein providers such as the Gates-sponsored Impossible Foods.
At the municipal level, thus far, the mayors of 14 cities around the world have signed the “C40 Good Food Cities Declaration,” committing to getting their citizens on the Planetary Diet by 2030. The cities—Barcelona, Copenhagen, Guadalajara, Lima, London, Los Angeles, Milan, Oslo, Paris, Quezon City, Seoul, Stockholm, Tokyo, and Toronto—will use their policy and procurement powers to steer citizens in the “right” food choice direction.
Sources:
“14 cities commit to sustainable food policies that will address the global climate emergency.” C40 Cities, Oct. 10, 2019.
“How was the EAT-Lancet Commission funded?” EAT. Accessed Nov. 15, 2022.
“The Planetary Health Diet.” EAT-Lancet Commission, 2019.
Joseph Mercola. “The real reason Bill Gates, Jeff Bezos and Google want you to eat lab-grown ‘meat.’” The Defender, May 12, 2021.
Whitney Webb and Jeremy Lofredo. “Developers of Oxford-AstraZeneca vaccine tied to UK eugenics movement.” Unlimited Hangout, Dec. 26, 2020.
Whitney Webb. “A ‘leap’ toward humanity’s destruction.” Unlimited Hangout, Jun. 25, 2021.
Indoctrination at School
Concerningly, there are worldwide efforts to steer food and food education programs in schools in the direction of pharma foods, for example, through coalitions such as the USDA-led and EU-endorsed School Meals Coalition, launched during the UNFSS.
In the Netherlands, the Wageningen University—a worldwide leader in promoting a technocratic food agenda—has provided, for the past 15 years, food and nutritional education programs to 75% of Dutch elementary schools. The university develops its programs in collaboration with the government, other educational institutions, businesses, and societal organizations.171 These education programs currently include an “adventurous tasting mission,” which, in the city of Zwolle, offered mealworms to schoolchildren as part of the lesson plan.172
In the U.S., the Rockefeller Foundation has called for public and private funding for school food programs. In a July 2020 report titled, Reset the Table: Meeting the Moment to Transform the U.S. Food System, the Foundation called for investing in “frontline community institutions including colleges, universities, and childcare centers that provide meals to children and youth and influence their dietary preferences.”173 To provide a rationale for their proposed programs, they cited the U.S. Census finding that from 28% to 33% of households with children “couldn’t afford to buy the amount or quality of food they wanted,” adding that in 16.5% of such households, “parents are directly reporting that their children are not getting enough food—meaning that nearly 14 million children are going hungry on a regular basis.”
Of course, nobody wants children to go hungry. But this scenario is pretty grim—after our governments have made it impossible for many parents to work and provide for their children, the same institutions are throwing out faux lifelines in the form of food “aid.” Parents who do not want their children to go hungry will send them to school to feed them, and in the U.S. they can sign up for food assistance programs such as the Supplemental Nutrition Assistance Program (SNAP), the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and the Child and Adult Care Food Program (CACFP). Corey Lynn warns about the caveats of these systems, observing that “It’s all about the SNAP Trap and WIC – getting everyone onto the free food system and into socialism and being controlled.” She continues:
“[It is about] monitoring and controlling food access and disbursement to provide genetically modified foods, gene edited foods, bioengineered foods, bugs and insects, and lab grown meat through channels we control, while utilizing the term health care to convince people it is all for their own well being.” 152
Citing an example from Illinois, Lynn shows that getting access to these services can entail signing up for a blockchained digital token system that controls the food choices that are available.
Rockefeller “Food Is Medicine” Initiative
As part of government food programs, the Rockefeller Foundation also advocates for a “Food is Medicine” approach.174 The approach, floated in 2020, proposes “linking together” food and health systems to “save both lives and money, and better protect against future pandemics.” The notable selling point is as follows:
“By integrating healthy food into the health care system, doctors could prescribe produce as easily as pharmaceuticals and reduce utilization of expensive health services that are often required because of nutrition insecurity.” 173
Lynn explains how these “food prescriptions” are taking shape, making it possible to prescribe medically tailored groceries and meals and order nutritional screening with the “necessary” support of biometrics and tracking.152 These initiatives could even entail personalized diets based on your genomic profile.99 The NIH has allocated $170 million to study how “precision nutrition” can consolidate information on “genetics, gut microbes, and other lifestyle, biological, environmental, or social factors to help each individual develop eating recommendations that improve overall health.”175
The ESG Investment Nudge
As already discussed, the alleged promise of lab-grown meat is that it will reduce environmental impacts of food production, ameliorate land use, and decrease the risk of zoonotic pandemics. In keeping with this narrative, GFI and the FAIRR network of investors focused on the risks of livestock agriculture have joined hands and introduced two ESG (Environment, Social, and Governance) reporting frameworks to account for the ESG impacts of synthetic proteins.176
With total assets under management of $70 trillion, FAIRR’s members include, among others:
- BlackRock
- Fidelity
- J.P. Morgan
- The Capital Group
- Morgan Stanley
- Credit Suisse
- Aegon
- Axa Investment Managers
- Allianz
- UBS
- Edmond de Rothschild Asset Management
- Rockefeller Asset Management
F. William Engdahl does not mince his words when he comments on the real goals behind the “false claim of ‘sustainable agriculture.’” He notes:
“[The fact that sustainable agriculture] is being driven by the world’s largest wealth managers including BlackRock, JP Morgan, AXA and such, tells volumes about the true agenda. These are some of the most corrupt financial institutions on the planet. They never put a penny where they are not guaranteed huge profits. The war on farming is their next target.” 153
In short, the goal of the ESG scoring is to shift institutional investments out of livestock farming and into synthetic alternatives. To add fuel to the fire, the FAO announced at COP27 that within a year, it will launch a blueprint to reduce emissions from agricultural production.
The ESG push is also putting an undue financial and administrative burden on small farmers and businesses. For example, the SEC, in March 2022, proposed to improve climate reporting by including disclosures about “Scope 3” emissions—“emissions occurring in the value chain of the reporting company”—in certain reporting requirements.177 If a small business or farm wants to do business with an organization that is registered with the SEC, it will need to adopt new environmental reporting standards. In addition, requiring these disclosures will force companies and farms to reveal sensitive private or business information.
As we have already seen, lab-grown meat is confronted with almost unsurmountable challenges; in a normal economy, even with unlikely technological advances, the price of lab-grown meat would never be competitive with real meat. However, ESG scoring and emissions taxes can change that equation. In this context, the comments of New Harvest’s CEO Isha Datar in an interview with journalist Joe Fassler are telling. Suggesting that measuring cultivated meat prices against current meat prices is an unfair comparison, Datar’s logic is that the “traditional” meat supply chain will “become more precarious and expensive as resources like land and water become scarce.” She remarked:
“We can’t really assume that animal agriculture is going to continue the way it has…. I can only see the price of cultured meat coming down, and I can only see the price of meat from animals going up, in a changing world.”64
IX. Regulatory and Cultural Hurdles
The Regulatory Frontrunners: Singapore and the U.S.
Until now, regulation has been a major bottleneck for bringing lab-grown meat to market. In most countries, synthetic meat is regulated under so-called “novel food” policies. Novel foods are foods and food ingredients—such as insects and nanomaterials—that most people historically would not recognize as food. Novel foods can also include new production methods, such as those used for pharma foods.
Singapore and the United States are currently the frontrunners. As already noted, Singapore is the only country in the world to have fully approved synthetic meat (Eat Just’s lab-grown chicken). The Singapore Food Agency evaluates synthetic meat products on a case-by-case basis.19 Safety assessments, ingredients, and other information from the dossiers of novel-meat producers are not publicly available, as these are considered confidential.
In the U.S., the FDA and USDA agreed in 2019 on a joint regulatory approval process for “cell-cultured meat.” The FDA is responsible for overseeing the first part of the process that includes cell collection, growth, and differentiation. The USDA then takes over and oversees harvesting and subsequent production steps, as well as providing labeling requirements.178 One account of the regulatory climate suggests that stakeholders from the synthetic meat sector preferred FDA oversight, considering the agency more “politically neutral” and more accustomed to regulating both food and pharma products.8 Although that may be the case, cell cultures have never been eaten—and the FDA’s existing cell culture guidelines are not designed for food. Furthermore, Jaydee Hanson at the Center for Food Safety warns that growth media components do not need to be fully disclosed; for ingredients with GRAS status (Generally Recognized as Safe), companies would be allowed to conduct their own safety assessments. Hanson disputes this loophole:
“Since companies are not required to fully disclose the composition of their scaffolding or growth media, potentially exposing consumers to novel proteins and allergens, the new mixture of ingredients should be reviewed under a full FDA supervised food additive review, not GRAS.”110
In November 2022, the FDA announced that it had granted pre-market approval for a lab-chicken product made by UPSIDE Foods, stating, “We evaluated the information UPSIDE Foods submitted to the agency and have no further questions at this time about the firm’s safety conclusion.”179 Hanson, however, does have some questions:
“[The FDA’s review] is … grossly inadequate. …[N]either the company nor the FDA presented the actual data from tests looking at the effects of raising these cells in fetal bovine serum and enzymes from the intestines and pancreas of animals. Likewise, while the company notes that it uses genetic engineering to keep the cells growing, it fails to share which genes are being used. This is vital information that consumers and policymakers need to know to make informed decisions in the best interests of public health. We should make certain that genes linked to cancer are not being used.” 180
As for precision-fermented products, the FDA approves them on a case-by-case basis. Many companies—such as Motif Foodworks, Perfect Day, the Every Company, and Nature’s Fynd—have already received “no further questions” letters from the regulatory agency for their synbio products.
Other Jurisdictions
Because the regulatory approval process differs by country, we discuss other selected jurisdictions separately below.
European Union
In the EU, pharma foods fall under Novel Food legislation. This legislation takes a risk-based approach and requires safety assessments by the European Food Safety Authority (EFSA) before allowing such foods on the market. Novel foods also require labeling information, including information on food characteristics. Post-marketing monitoring may also be required.
Once EFSA has granted a positive safety review, it sends the dossier for approval to the Standing Committee on Plants, Animals, Food and Feed (PAFF). The committee has representatives from all member states; to get approval, a 55% majority must vote in favor, and this majority must make up 65% of the EU population.181 The EU bureaucracy may present more obstacles for the regulatory approval of synthetic meat than elsewhere—if the production process uses genetic engineering (for example, for the cell lines or culture media), then the EU’s GMO legislation also applies.14 In addition, several EU guidelines and regulatory requirements prohibit or discourage the use of fetal bovine serum, including the Good Cell Culturing Principles (GCCP), the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) legislation, and—depending on how the FBS is made—regulations to protect animals used for scientific purposes.15 As of November 24, 2022, no dossiers had been filed for approval of lab-grown meat.
One would expect that precision-fermented products fall under the EU’s strict GMO legislation. This is not the case, however; because GMO bacteria are considered “processing aids,” they fall outside the scope of GMO regulation. The relevant EU regulation states:
“Processing aids which are only used during the food or feed production process are not covered by the definition of food or feed and, therefore, are not included in the scope of this Regulation. Nor are food and feed which are manufactured with the help of a genetically modified processing aid included in the scope of this Regulation.”182
Thus, precision-fermented products have been approved under the EU’s novel food policy and are sold on the EU market without adequate labeling. For example, EFSA has approved 2′-fucosyllactose (2′-FL),183 a substance that resembles components of human milk. This component is recognized as GRAS in the U.S.184 It can be “produced by chemical synthesis or with the authorized microbial sources of genetically modified strains of Escherichia coli,”185 and is used in infant formula and food supplements for children.183
Five types of insects have been approved as food under the EU’s Novel Food policy:186,187
- Acheta domesticus (house cricket)
- Gryllodes sigillatus (dried tropical house cricket)
- Locusta migratoria (locust)
- Tenebrio molitor (dried mealworm)
- Alphitobius diaperinus in larval form (lesser mealworm)
United Kingdom
The UK follows novel food approval procedures that are very similar to those of the EU. A company can submit a dossier for a novel food to the UK Food Standards Agency. However, the final decision on approval lies not with a committee but with government ministers. There is a possibility that the UK may develop a separate framework for approval of synthetic meat.188
Israel
In Israel, the National Food Service, operating under the Ministry of Health, approves novel foods on a case-by-case basis. The novel food assessments conducted thus far have not been tailored to the synthetic meat production process. The Ministry of Health has initiated research into the safety assessments of synthetic meat to determine criteria that are specific for those products.188
China
According to the GFI, “China has not yet announced how it will regulate or oversee the manufacturing and sale of cultivated meat.”19 In a presentation on the regulatory environment for lab-grown meat, Leatherhead Food Research detailed China’s novel foods application process, which is very similar to the process followed by the EU.189
Japan
The GFI reports that cell-cultured meat is likely already legal under existing legislation in Japan, provided that no immortal cell lines or growth factors are used.188 However, to provide clarity and ensure consumer safety, the Center for Rule-making Strategies is working with a public-private partnership—the Japan Association for Cellular Agriculture (JACA)—to develop industry guidelines and create a proposal for regulation of synthetic meat.190
Brazil
Brazil is in the process of developing a regulatory framework for lab-grown foods. GFI in Brazil has lobbied for a specific protocol for synthetic meat that would fall under Brazil’s existing novel food regulations. The Brazilian Health Regulatory Agency, Anvisa, has indicated that it will follow a model resembling that of the EU and the U.S.19
What’s in a Name?
What to call cell-cultured meats and other pharma foods once they are allowed on the market is a topic of much contention. Clearly, cell cultures do not fall under existing definitions of meat in the U.S. or EU:
- In the U.S., the USDA defines meat as “the flesh of animals (including fishes and birds) used as food, that can be part of a healthful diet.”
- In the EU, the Food Information to Consumers regulation defines meat as “skeletal muscles of mammalian and bird species recognised as fit for human consumption with naturally included or adherent tissues.”
In September 2021, the USDA requested public comments about how to label lab-grown meat products.191 Not surprisingly, GFI argued that USDA should “adopt a practical and flexible regulatory framework that permits cultivated meat companies to use familiar terms, avoids rigid and premature regulation of terminology, and ensures a fair playing field for cultivated meat and poultry.19 The Center for Food Safety took the opposite position, emphasizing that consumers must be able to make informed decisions:
“The USDA must ensure that the labels used on these products distinguish the cell-derived imitations from real meat and poultry. USDA should use a label like ‘synthetic protein product made from beef cells.’ We don’t allow artificial vanilla to be called vanilla, but rather call it synthetic vanilla. A similar naming is called for here to avoid confusion.”192
Market research shows that consumers’ willingness to try these foods is very much affected by how they are named. “Lab-grown” is a deterrent, while terms like “clean” and “slaughter-free” are more appealing.15 In a sense, the synthetic meat industry is getting caught up in its own semantics. The products are supposed to be “exactly like real meat,” while simultaneously the industry is promoting them as the leading edge of FoodTech innovation. In the end, though, what is attractive to transhumanist VC investors is not so appetizing for consumers.
Battles about which foods are allowed to be labeled as “meat” are already being fought in European and American courts. In 2019, for instance, after the French parliament passed a bill that restricted the use of labels such as “meat,” “milk,” or “sausage” to products of animal origin,193 the German company Tofutown fought the decision at the European court. The court decided that “sales designations for dairy products cannot be used to market purely plant-based products, because EU regulations define ‘cheese,’ ‘milk’ and similar designations as coming from an animal.”15
In 2018, the U.S. state of Missouri passed a similar bill. Tofurky and GFI have challenged it in court—thus far unsuccessfully. The GFI calls these types of bills “label censorship” and, together with alt-protein companies, it is suing other states such as Louisiana, Arkansas, and Oklahoma.19
A Back Door: Codex Alimentarius
The Codex Alimentarius is a UN body founded by the FAO and WHO in 1963 to develop standards for safe foods in order to facilitate trade. As a standard-setting organization, it does not have the power to legislate, but in practice, this works out differently. In her article “Inside Codex with Scott Tips: New Global Food Diet – Insects, Rats and Dogs,” Corey Lynn explains that the 189 UN member-states translate the Codex standards into legislation. She writes that the USDA “is a driving force for not only adhering to the standards, but making certain other countries follow in lock step as well.”150
The WHO foresees a key role for Codex in creating alignment on new foods such as alternative plant-based and pharma-produced proteins, insects, rats, foods featuring nanotech, and more.150 To help build “global unity around alternative proteins,” the GFI joined Codex in February 2021.194 Being granted official observer status at Codex means that GFI can “now contribute to establishing an international regulatory framework for alternative protein production.”195 Unfortunately, this is a perfect avenue for the lab-grown meat lobby to overcome a lot of legislative barriers at once. Though few people may even be aware of the existence of Codex, once a consensus has been established at this level, resistance at local levels becomes far more challenging.
Another Back Door: Pets and Livestock
Alt-protein providers have not just set their sights on human consumers; another target market is pets. In the U.S., pet foods are a $30 billion market. According to Pitchbook, at least 15 VCs are investing in “pet technology” such as “meat-free kibble.”196 In 2022, for example, the VC firm Agronomics, together with Roslin Technologies (of Dolly the cloned sheep fame), launched Good Dog Food to produce cell-cultured pet food.197
The Economist assumes that this market is driven by vegan pet owners desperately looking for animal-friendly foods, also assuming (and ignoring research on species-appropriate diets) that dogs and cats may not be too picky about the meats they are served.198 But the real reason for the forays into lab-cultured pet food may be more practical. The company Wild Earth—helped by funders such as Peter Thiel’s Founders Fund (which also invests in SpaceX, Palantir, and Facebook)—spells out that they will use mouse cells to develop their products, a choice that would solve a number of basic problems all at once.199 Well-characterized mouse cell lines that are relatively easy to culture are publicly available. In addition, regulation and oversight of pet food production may be less stringent than for human foods.
Where cell-cultured foods for pets are still on the drawing board, another type of alt-protein pet food is already being sold: insect-based pet food. The largest insect factory in the world, Protix, produces insect-based pet food as well as feed for livestock and aquaculture, selling these products in 12 countries.200 This is probably not without risk; in a 2018 study that investigated insects at 300 farms and pet stores, the researchers found parasites at 244 of them.201 The investigators estimated that these parasites could pose a danger to 35% of animals and 30% of humans. It is widely recognized that pets can be a vector for parasites to humans. “Pets can carry parasites and pass parasites to people,” warns the CDC.202 Therefore, feeding pets with parasite-infested insects, and especially pets that do not have the physiology to digest bugs, may not be such a good idea.
The climate narrative has migrated into the pet arena, with news outlets speaking of “carbon pawprints” and portraying pets and their foods as major contributors to climate problems. According to a CNN report, “[F]eeding dogs and cats creates the equivalent of around 64 million tons of carbon dioxide in the US each year. That’s roughly the same impact as 13.6 million cars on the road.”203 To reduce the climate impact of your pets, ESG scoring on pet foods is likely to direct you to meat alternatives.
Popularizing Cannibalism?
Linking back to lab meat’s early hints in the art world, the corporate media regularly float stories suggesting that cannibalism is in some way “cool.” Influenced by the newer technologies, the current messaging also implies that if human meat were to come from cell lines—and not from a dead body—it would not, in theory, be cannibalism.
In December 2020, for example, the London Design Museum displayed an “art project”—presenting a concept for a DIY kit to culture human cells with expired blood and grow an “Ouroboros Steak” at home.204 One of the artists clarified, “People think that eating oneself is cannibalism, which technically this is not.” A few years before, in 2017, the start-up BiteLabs proposed growing synthetic meat using cells from celebrities, apparently assuming that growing “Ellen DeGeneres salami” might increase the appeal of synthetic meat.205,206
Scientists are also on board. The fanatic atheist Richard Dawkins suggested in a 2018 tweet, “What if human meat is grown? Could we overcome our taboo against cannibalism? An interesting test case for consequentialist morality versus ‘yuck reaction’ absolutism.”207 The next year, Swedish behavioral scientist Magnus Soderlund stated on national television that climate change could force people “to try foods they currently wouldn’t think of eating,” giving the examples of “pets, insects, and human flesh.” According to Soderlund, we must “awake the idea” of eating human flesh.208
Strangely, cannibalism also seems to be a popular and recurring theme in mass entertainment. The online publication Vigilant Citizen, which tracks satanic messaging in the media, describes many series and movies, mostly on Netflix, that deal with cannibalism. These include Chilling Adventures of Sabrina (“an American supernatural horror series”), Brand New Cherry Flavor (an “American horror-drama” and “mind-altering journey of supernatural revenge”), and the Santa Clarita Diet (an “American horror-comedy” featuring Drew Barrymore).209,210
In 2022, the New York Times wrote an article titled “A Taste for Cannibalism?”, quoted by LifeSite News as stating: “Turns out, cannibalism has a time and a place. In the pages of some recent stomach-churning books, and on television and film screens, Ms. Summers and others suggest that that time is now.”211 The author in question, “Ms. Summers,” wrote a novel celebrating cannibalism titled A Certain Hunger. The National Review followed up with the article, “Cannibalism Chic?”212 outlining even more series, books, and movies dealing with eating human flesh; the author concluded, “The unthinkable is not only thinkable, but the new 50 Shades of Grey?”
For most consumers, meat from human cell lines would make the already appalling prospect of eating cell-culture meat even more disgusting. From the industry’s standpoint, however, this approach would—just like with the mouse cells in the pet food market—solve numerous practical problems. Notably, human cell lines are publicly available, together with data about their characteristics and how to culture them.
In her article “Cool Cannibals,” author Karen Hunt compares stem cell research and therapies and fetal tissue harvesting to the superstitious child sacrifice practices of African witch doctors and the Aztec, Incan, Mayan, Moche, and Timoto-Cuica cultures—and even of the Christian Pope Innocent VIII in 1492. The rituals around them may have changed, but boiled down to their essence, the practices are the same: innocents are sacrificed to assure the health, prosperity, and longevity of the rich and powerful. In 2021, the U.S. hosted 3,000 unregulated and experimental stem cell clinics, an increase of 2,400 compared to 2016.213 It appears as if—as long as an activity is pursued as “science”—anything goes, with no moral boundaries.
Sacrifices justified by a pseudo-science that cloaks itself in a mask of rationality may be more dangerous to the common man than the “entertainment” media’s tongue-in-cheek presentations of cannibalism. In the latter, it is possible to assume it is a joke and keep it in the domain of fiction. But in the science realm, normal people are willing to participate because—just like in the notorious Milgram experiments—the authority of science has eclipsed the personal duty to evaluate the situation’s ethics.
X. Unanswered Questions: Why the Big Push?
Behind-the-Scenes Motivations
Looking at the formidable technical, financial, and regulatory challenges that synthetic meat faces—as well as the marked lack of consumer interest—it is necessary to ask why there is such a push to develop it on a large scale. When looking purely at the economics, these investments do not make sense. No matter what innovations come along, synthetic meat production will remain a complicated, high-tech, and capital-intensive process—for a low-margin product. If a company succeeds in setting up such a process, why use it to make a product with the profit margins of a hamburger instead of a biotech medicine? And unlike with other technological investments, even if consumers adopt synthetic meat at a mass scale, the profits will never show a hockey-stick growth curve. Ordinarily, these factors would make this sector an unlikely candidate for VC money.
Below, I explore various alternative motivations that could be driving the investments in lab-grown meat and other pharma foods.
Control of Food Is Control of People
As policies and events during the central-banker-orchestrated Going Direct Reset have made clear, the end goal of the reset is to have each individual tagged or chipped and managed with a social credit and behavioral score. A controlled food system and an engineered food crisis may be a necessity to get enough people to sign up for digital credit voluntarily.
Deliberate policies to control people through food are not new and have unfolded in several waves via the Green Revolution, the Gene Revolution, and now, through FoodTech. In his book Seeds of Destruction: Hidden Agenda of Genetic Manipulation, F. William Engdahl documents the transformation of agriculture in the U.S. and abroad through 2007—outlining the transition from resilient decentralized systems of food production made up of self-sufficient smallholder farmers into a centralized system dominated by a handful of agribusiness corporations in which the farmer is little more than a serf. (The book, well worth reading, is available at .)
This agricultural transformation—formulated with the explicit goal of controlling people through control of food—was part of a deliberate strategy developed by Rockefeller protégé Henry Kissinger, backed by Rockefeller money and carried out through U.S. foreign and domestic policies. Moreover, as Engdahl documents, the efforts to control the food supply were intimately tied to eugenics goals to control and reduce population growth. As Secretary of State and National Security Advisor under Nixon, Kissinger outlined a strategy for world population control in top-secret National Security Study Memorandum 200 (NSSM 200) titled “Implications of Worldwide Population Growth for US Security and Overseas Interests.” A key lever to enforce the policy suggestions contained in this memo was “food as a weapon.”
Wave 1: The Green Revolution
The first wave in the move to control food production was the so-called Green Revolution, which claimed to apply a more “scientific” approach to agriculture to make production more “efficient.” However, “innovations” such as monocropping—boosted by fertilizers and pesticides—and moving livestock into industrial-scale animal factories led to the problems that are now associated with commercial non-organic agriculture: depleted soils, biodiversity loss, nitrogen pollution, pesticide overuse, animal waste, and animal suffering.
In fact, the Green Revolution’s new “efficiencies” severed all the natural connections that bind animals, plants, and people. In addition, family-owned farms turned into megafarms that needed fewer employees, and in which the farmer was largely a contractor to agribusiness corporations instead of an independent entrepreneur. In Seeds of Destruction, Engdahl writes (p. 138):
“Between 1979 and 1998, the number of US farmers dropped by 300,000. […] A report to the US Secretary of Agriculture in the late 1990s described the enormous social costs of the destruction of the American family farm by agribusiness, as the economic basis of entire rural families collapsed and rural towns became ghost towns.”
The effect of these policies in developing countries was even worse. According to Engdahl (p. 128):
“One major effect of the Green Revolution was to depopulate the countryside of peasants who were forced to flee into shantytown slums around the cities in desperate search for work. That was no accident; it was part of the plan to create cheap labor pools for forthcoming US multinational manufacturers.”
Wave 2: The Gene Revolution
The second wave of consolidation of the food supply came with the “Gene Revolution.” Engdahl details (p. 94) how genetic science as we know it today emerged out of eugenics “science” and was 100% funded by the Rockefeller Foundation. After Nazi atrocities made eugenics unpopular among the general populace, it was disguised as “genetics” and further pursued by the American Society of Human Genetics (later, a sponsor of the Human Genome Project). Genetics takes reductionism and materialism to the extreme, reducing life to traits that are carried by genes. Consequently, this perspective on life assumed that all problems could be solved by manipulating genes.
Identifying species by their genetic traits was pivotal for getting control of global seed supplies and patenting GMO seeds, a project that carries on to this day.147 At the same time, the Gene Revolution created yet another cost burden for farmers, who now had to pay royalties for the patented GMO seeds. Though the seeds were marketed as being pest-resistant, in practice, they required more pesticides because of their tendency to create resistant weeds and pests.
In addition, as Engdahl documents (p. 240), it soon became apparent that GMOs were very unhealthy to eat. In 1998, then world-renowned geneticist Arpud Pusztai—shocked by what he found when he studied a GMO diet in rats—shared his discoveries on British television. He reported that rats fed GMO foods for more than 110 days had smaller liver and heart sizes, smaller brains, and weakened immune systems. Pusztai’s case is one of the earlier examples of character assassination of scientists with a conscience. His disclosures led to political interference at the highest levels; within 48 hours, Pusztai lost his research contract, followed by a disinformation and smear campaign refuting his claims. Further research into GMO safety was actively suppressed. In the U.S., where the FDA followed a policy of industry self-regulation, GMOs flooded the market with no labeling and without informing the public of the risks.
Wave 3: FoodTech and the Digital Takeover of Food
Engdahl’s book may have ended in 2007, but if we follow his line of history into current developments, we can recognize current FoodTech trends as the third wave of consolidation and control of food production. This wave is characterized by genetic manipulation run amok and technologies that leverage innovations in computing power, including storage capacity for big data and the more powerful computing and analysis enabled by algorithms and AI. Both serve to further centralize and control the means of food production.
Technocracy expert Patrick Wood warns that this constitutes a “genetic takeover of all living things” and points to the UN’s Biodiversity Convention of 1992 as the blueprint for this takeover. This convention laid the groundwork for the capture of “biodiversity”—defined by its “genetic resources”—to allow the biotech sector to patent it.214 Recently, UN working groups preparing an update to the 1992 Convention replaced the term “genetic resources” with “digital sequence information on genetic resources.”215 This conveys the intent to reduce the richness and abundance of life to binary codes to open them up for appropriation and exploitation.
The newly launched Wall Street asset class called a “Natural Asset Company” (NAC) may serve as the perfect vehicle to capture ownership of “digital sequence information on genetic resources” in wide swaths of land. A NAC separates what is produced by nature on a piece of land—including clean water, air, food, and even biodiversity—from the land itself and brings these into the NAC. The Wall Street argument is that these vehicles will attract funding for environmental causes that is difficult to attain in other ways. For the more cynical observer, however, the NAC is a way to take away people’s right to live on and from the land.216
In light of the digital-genetic takeover, it is worth noting the massive push for genomic surveillance. Global disease surveillance networks are a centerpiece of the WHO’s One Health framework (see Figure 13), including multiple WHO programs to surveil “pathogens with pandemic and epidemic potential.” This genetic surveillance also applies to food production.217-219 Under the banner of One Health, genomic surveillance capacity is being expanded to people, plants, animals, and nature—and these databases are being connected. The interest in surveillance is not new, however. In 2007, the Rockefeller Foundation launched a Disease Surveillance Network (DSN) initiative, with an initial investment of $22 million. In the subsequent year, the One Health Commission was born, thanks to a “significant” but unspecified donation from the Foundation.
Figure 13. One Health Framework
Pharma Food Dual Use?
We have seen that synthetic meat uses the same production processes as biotech pharmaceuticals. This raises a pressing question: Could the new “meat” also be used as a delivery system for biopharmaceuticals? The question is not far-fetched. Consider the examples of “edible vaccines” and hydrogels:
- Edible Vaccines: Edible vaccines are currently the focus of research.220 A grant of $500,000 from the National Science Foundation (NSF) to the University of California-Riverside is funding research on whether “DNA containing the mRNA vaccines can be successfully delivered into the part of plant cells where it will replicate, demonstrating the plants can produce enough mRNA to rival a traditional shot, and finally, determining the right dosage,” so that vaccination will “look more like eating a salad than getting a shot in the arm.”221
- Hydrogels: Hydrogels, which are a candidate for creating the structure of synthetic meat, are also used for drug delivery. Specifically, they can be used in combination with lipid nanoparticles (LNPs),222,223 which are also used for mRNA delivery in the Pfizer/BioNTech, CureVac, and Moderna injections.224 Profusa, a company funded by Bill Gates and DARPA, develops hydrogels that function as implantable biosensors.225,226
In Seeds of Destruction, Engdahl consistently lays out the connections between genetically engineered foods and eugenics policies for population reduction. Already in the 1990s, scientists and others had recognized the dual-use potential of biotech research. Engdahl cites the assessments of Lt. Col. Robert P. Kadlec about the biowarfare potential of GMO crops:
“Compared with other mass destruction weapons, biological weapons are cheap. A recent Office of Technology Assessment report places the cost of a BW [biological weapon] arsenal as low as $10 million … in stark contrast to a low-estimate of $200 million for developing a single nuclear weapon. […] Using biological weapons under the cover of an endemic or natural disease occurrence provides the attacker the potential for plausible denial. In this context, biological weapons offer greater possibilities for use than do nuclear weapons.”
Transhumanism and the Quest for Eternal Life
The connections between synthetic meat and regenerative medicine (tissue engineering) are well documented. For example, the CEO of Israel’s Aleph Farms, Didier Toubia, explained in an interview with Business Insider: “The key for us is the access to that science of regenerative medicine. That gives us a significant edge.”227 After cloning Dolly the sheep, the Roslin Institute in Scotland became an expert in creating stem cells that could be used in regenerative medicine.228,229 Currently, its expertise is in creating cell lines for cell-cultured meat production.
In 2021, the GFI joined the Advanced Regenerative Manufacturing Institute (ARMI) to “encourage cross-pollination between innovators in the cultivated meat and regenerative medicine sectors.” ARMI was founded in December 2016 with $80 million in funding from DOD and an additional $214 million from industry partners. ARMI’s aim is to “make practical the scalable, consistent, and cost-effective manufacturing of cells, tissues, and organs and develop the trained and ready workforce necessary for that manufacturing.”230
The field of regenerative medicine also has received ample funding—$4.4 billion in the last five years—from billionaires seeking to prolong their lives. Despite the billions invested, however, the longevity field has seen little progress. Billionaire Christian Angermayer explained the motivations for these investments during the exclusive Longevity Investors Conference:
“If you buy a yacht, you can always get a bigger yacht; if you buy a plane, you can always get a bigger plane. But the [extent to which] your life is changing with more money is actually very minimal. It makes more sense to direct funds to being healthier and living longer.” 231
Is the surge of investments in cell-cultured meat generating research, collaborations, and other investments in a way that purely financial injections cannot? Stem cell researcher Stephanie Wallis poses the question in a TEDx talk titled “Could Cultivated Meat Fast-Track Regenerative Medicine?”232 It may be easier to attract passionate support from idealistic people if you tell them they are saving the world from the dangers of animal meat and climate change with their research, versus buying off scientists to make the ultra-rich live longer.
Cellular Agriculture Australia writes, “Currently, most … stem cell and regenerative medicine knowledge is not focused on developing cultivated meat, but some experts are starting to shift from academia into the cell ag industry.”233 The cross-pollination is also evident in the funding for the research article “Cell Sources for Cultivated Meat,” which came partially from the University of Wisconsin Stem Cell & Regenerative Medicine Center.111 Meanwhile, the leader of Mosa Meat’s cell biology team explains that one of the company’s key challenges is “trying to understand why cells age,”114 a quest shared by regenerative medicine researchers. The CEO of Matrix Meats notes, “The technology that we use has also been used in regenerative medicine for 15 years.”127 Matrix Meats licenses its technology from Nanofiber Solutions, a company with multiple research contracts from DOD, NIH, and the NSF.234
Interestingly, the billionaires and VCs who are funding lab meat are also investing in longevity research. For example:
- Jeff Bezos—with investments in the VC Breakthrough Energy Ventures and in companies such as Wildtype, Ginkgo Bioworks, and Beyond Meat—is a major investor in the “life extension therapy” company Altos Labs. Bezos has also funded Unity Biotechnology, which develops life-extending technology.235,236
- Altos Labs, in turn, is run by founder and chief scientist Rick Klausner, a former Executive Director for Global Health of the Bill & Melinda Gates Foundation237 and an investor in the synthetic meat company Meatable.238
- Another funder of Unity is Peter Thiel, who—in addition to funding synthetic meat and space journeys—is heavily invested in life-extending activities, such as the SENS Research Foundation that “reimagines aging” and the Methuselah Foundation, “a non-profit medical charity focused on extending the healthy human lifespan by making 90 the new 50 by 2030.”239
- Sergey Brin, who financed the world’s first lab burger, together with Larry Page co-founded Calico Labs, a “secretive company that’s seeking to extend lifespan through genetic research and drug development.”240
- In 2016, Jim Mellon founded Juvenescence, a company that offers a wide range of anti-aging therapies. He currently leads the Roslin Technologies and Agronomics joint venture Good Dog Food.197,241
- Khosla Ventures, also an Eat Just investor, funded Cellino, regenerative medicine with AI-based stem cell culturing technology.
Geophysical Risks?
Under what circumstances would it be rational to bring all food production indoors, independent of natural systems? If world leaders anticipate major geophysical risks to Earth—and the risks would make it difficult to grow food and raise livestock outdoors—then it could make sense to experiment with synthetic foods and systems such as vertical indoor farms.
In this regard, it is interesting that the person who gave the lab-meat field a kickstart, Jason Matheny, started his career at the Future of Humanity Institute at Oxford University. In an article from 2007,242 he lists catastrophes that pose “near-term” extinction risks [list format added]:
- “[N]uclear detonations, asteroid or comet impacts, or volcanic eruptions could generate enough atmospheric debris to terminate food production;
- [A] nearby supernova or gamma ray burst could sterilize Earth with deadly radiation;
- [G]reenhouse gas emissions could trigger a positive feedback loop, causing a radical change in climate;
- [A] genetically engineered microbe could be unleashed, causing a global plague; or
- [A] high-energy physics experiment could go awry, creating a ‘true vacuum’ or strangelets that destroy the planet.”
On a longer time horizon, Matheny observed that “self-replicating nanotechnologies could destroy the ecosystem; and cognitive enhancements or recursively self-improving computers could exceed normal human ingenuity to create uniquely powerful weapons” (see “Matheny’s Bold Solutions”).
We might add to this set of risks the possibility of a Maunder Minimum—a decrease in sunspot activity that could cause a new Little Ice Age.243 In addition, the magnetic pole is moving, Earth’s magnetic field strength is decreasing,244 and the atmosphere is losing oxygen.245 It is unclear what exactly is causing these changes and what their impacts on life on Earth may be.
Other unanswered questions concern the geoengineering experiments that are well underway. How do the cross-domain bacteria that Clifford Carnicom has found for decades in samples from all over the world relate to geophysical risks? Are the geoengineering experiments geophysical risks by themselves, or are the experiments designed to anticipate risks?
Matheny’s Bold Solutions
In his 2007 paper, Jason Matheny proposed two bold solutions to anticipate geophysical and other risks and ensure humanity’s survival: underground bunkers and space colonies. He wrote:
“If we survive the next century, we are likely to build self-sufficient colonies in space. We would be motivated by self-interest to do so, as asteroids, moons, and planets have valuable resources to mine, and the technological requirements for colonization are not beyond imagination. Colonizing space sooner, rather than later, could reduce extinction risk, as a species’ survivability is closely related to the extent of its range. Citing, in particular, the threat of new biological weapons, Stephen Hawking has said, ‘I don’t think the human race will survive the next thousand years, unless we spread into space. There are too many accidents that can befall life on a single planet.’ Similarly, NASA Administrator, Michael, recently remarked: ‘The history of life on Earth is the history of extinction events, and human expansion into the Solar System is, in the end, fundamentally about the survival of the species.”
Matheny continued:
“Perhaps more cost effective than building refuges in space would be building them on Earth. Elaborate bunkers exist for government leaders to occupy during a nuclear war. And remote facilities are planned to protect crop seeds from ‘nuclear war, asteroid strikes, and climate change.’ But I know of no self-sufficient, remote, permanently occupied refuge meant to protect humanity from a range of possible extinction events” [emphasis in original].
Source:
Jason G. Matheny. “Reducing the risk of human extinction.” Risk Anal. 2007;27(5):1335-1344.
We are now coming full circle back to NASA’s project over two decades ago to use cell cultures to produce food during space journeys. In the last two years, growing synthetic meat in space has gained renewed attention. The Israeli synthetic meat company Aleph Farms even has its own space program.246 In April 2021, the company sent a team to the International Space Station with a SpaceX rocket to test if cells can be cultured in zero gravity; the researchers deemed the test successful.
In July of the same year, the European Space Agency (ESA) requested proposals for investigating whether synthetic meat can be grown in space, because cows would not thrive on the moon’s microgravity field. ESA engineer Christel Paille explained: “So, if we want to succeed in long-term human exploration far from Earth, we need to rethink our current approach to astronaut nutrition and provide the means to efficiently produce food on board, possibly integrated within the regenerative life support system.”247
XI. Take Action
Know Your Farmer and Boycott Big Ag
Perhaps the most important step that you can take to resist the ongoing assault of the Going Direct Reset is to ensure your food independence and your health. The current food system is dominated by oligopoly cartels that are shifting food supplies toward toxic, synthetic food. And the same regulatory parties that have been green-lighting toxic injections are overseeing the safety of the pharma foods now entering the market. It remains important to engage with your state and federal legislators about approval of new foods, but if you want to make sure you are eating non-toxic food, you should buy it directly from a source you trust.
Find a Farmer
If you can, try to find local farmers from whom you can buy your meat and produce directly. Attorney and food expert Pete Kennedy, host of Solari’s Food Series, has put together a wonderful list on “Finding Sources of Fresh Food,” included in this Wrap Up.248 The Food Series and other Solari series also offer numerous other resources, also listed in this Wrap Up.249
Grow Your Own Food
History is rife with cautionary tales about the dangers of centralized food production. For example, Stalin’s policies to collectivize agriculture in 1929 led to the Holodomor, the great famine that raged from 1931 to 1934 not only in Ukraine but throughout the Soviet Union. According to the Encyclopedia Britannica, at least five million people starved during this period, most of whom lived in Ukraine.
But the opposite is also true; there are beautiful examples of how food resilience has helped people thrive throughout dire, destabilizing world events. As the Solari team points out:
“World War II Victory Gardens grew within two years to provide as much as 40% of the US food supply. Gardening proficiency takes a few years of practice when starting from scratch. You don’t need a green thumb to enhance your food supply while living a free and inspired life.”250
Dachas (garden plots in the countryside owned by urban Russians) helped the population survive the ravages of the two World Wars and the collapse of the Soviet Union in the 1990s. According to Stephen Scott, co-owner of the heirloom seed company Terroir Seeds, “Dacha gardening accounts for about 3% of the arable land used in agriculture but grows an astounding 50% by value of the food eaten by Russians. According to official government statistics in 2000, over 35 million families (approximately 105 million people or 71% of the population) were engaged in dacha gardening.”251 Dacha gardening is deeply ingrained in the Russian culture and is a carrier of culture and community. The gardens provide an abundance of produce that food producers share generously within their communities.
In Nicaragua, people survived a regime change assault by the U.S. in 2018 because of a decentralized food system. In an interview with De Andere Krant, human rights lawyer Dan Kovalic shared a story:
“Nicaragua is almost 100% self-sufficient. They had food to eat because they grow food locally, not depending on the global market. And then we come back to the Sandinistas. They were primarily an agrarian movement, focused on making land available to farmers. Hundreds of thousands of peasants were given land, given free education and health care. In addition, they gave goods and livestock to the peasants. I visited a cooperative once, and they showed off a huge pig, explaining ‘we got it from Ortega.’ They are so food-independent that they even sent food to Cuba and Venezuela. That also makes it harder for the US—they can stop essential goods, like medicine, but they can’t starve the population through sanctions.” 252
Two low-threshold methods for growing your own food and increasing your food independence include the Square Foot Gardening Method,253 which only requires a 2×4-foot or 4×4-foot area in a sunny spot, and “guerilla gardening”—planting edible vegetables, fruits, and flowers in public spaces.
Save and Support Farmers
Many farmers are in a debt trap, and dependence on industrial agricultural inputs further reduces their profit margins. The “solution” being offered is to sell their farms. In the Netherlands, where the government is planning to buy out 3,000 farms, many are slowly doing so, one by one.254 In the U.S., Bill Gates, who already is the country’s largest landowner, continues to buy up more land.255 For each individual farmer, the decision to sell may be the most rational option, given the bureaucratic assault they are facing. To resist the takeover of our food systems by governments and Big Ag, it may be necessary to develop strategies to save farmers. Find out which farmers in your area are in need, and bring the community together to identify creative ways to refinance them. For farmers, it can be very helpful if you buy from them directly. Each intermediary in the supply chain lowers their profit margins.
XII. Conclusion: Stay Away from Toxic Pharma Food
As we noted in the introduction to this report, ensuring access to safe, high-quality, unadulterated food is not a new problem, with food additives, first-generation GMOs, pesticides, and undisclosed ingredients representing challenges to be navigated. However, lab-grown meat and other synthetic foods take these dangers to an entirely new level.
The safety and ethical questions raised by the invasion of Pharma Food are profound and include concerns about the absence of proper testing, industry players with negligent or even criminal records, untrustworthy regulators, and slippery labeling, not to mention even more disturbing concerns about the use of fetal materials and wholesale human experimentation and poisoning. The backdoor introduction of these synthetic items through the pet food and livestock feed supply chains, as well as the potential for a food-pharma merger in the form of new vaccines and medications also ring alarm bells.
Ultimately, each person must establish their own process for ensuring the quality control and integrity of their food. With regards to pharma meat, the solution, for now, is simple: Singapore is the only nation where a lab-grown meat product is available to consumers. However, the FDA’s premarket approval of UPSIDE Food’s pharma-chicken nuggets suggests it may not be long until the U.S. market offers these for sale. (For valuable additional suggestions on how to avoid fake food in the U.S. and EU, see the Appendix.)
As this report attempts to make clear, the “mad science” technologies of the biopharma industry and the full “transhumanist toolbox” have been unleashed on the entire food supply—and an ever-expanding array of “novel foods” is taking hold in local grocery stores while the companies that make them pop up in brokerage accounts. With the U.S. leading the way in bringing many of these foods to market, and the EU likely to serve as the next gateway jurisdiction, we can unfortunately expect many other countries to follow suit.
Appendix: Avoiding Pharma Food in the U.S. and European Union
The U.S.
In February 2019, a National Bioengineered Food Disclosure Standard went into effect in the U.S. The requirements for the “bioengineered” label are so convoluted that it appears it was designed to confuse.256 Although it would make sense to assume that the standard covers products to which genetic engineering has been applied, this is not the case, for many GMO products fall outside of the label’s requirements, including:
- Animals that have been fed GMOs
- Products that are so hyper-processed that the GMO can no longer be identified, such as sweeteners and refined oils
- Crops that do not contain detectible genetic material
- Gene-edited products (because genetic detection kits are largely lacking, these would be excluded from the labeling scheme)
Moreover, companies are free to choose between a set of options about how to signal that their products contain bioengineered ingredients: they may use a symbol, or the text “bioengineered food,” or simply place an electronic or digital link, such as a QR code or text message. If the product contains GMO ingredients that do not fall under the definition “contains detectable genetic material that has been modified through certain lab techniques and cannot be created through conventional breeding or found in nature,” a company may choose to self-report. It is now illegal, however, to call a product “GMO.”
Are you still following? It is probably best to avoid any foods that signal that they are bioengineered (see “Terminology to Avoid”). If you wish to ensure that the products you buy are non-GMO, both the “organic” label and the “non-GMO Project” label still exclude GMOs—whether traditional or GMO 2.0—from their labeling schemes.257 However, Max Goldberg, founder of Organic Insider, writes that on multiple occasions it has been suggested that GMOs and new genomic techniques be accepted under the organic labeling scheme, most recently at the National Organic Standards Board (NOSB) in the fall of 2022.258 Similar lobbying is taking place in the EU. To safeguard the integrity of organic and non-GMO labeling, Goldberg recommends that the NOSB meetings be much better attended by the organic industry, as the 15-member NOSB board is in a position to suggest that the USDA allow gene-editing technologies into the organic food supply.
For non-certified products, the following brands already use or create precision-fermented ingredients for ice cream, cream cheese, sweets, milk, and other beverages sold as “natural” and “animal-free”:259
- Modern Kitchen
- Bold Cultr
- Perfect Day
- The Urgent Company
- Brave Robot
- Nick’s
- California Performance Co.
- Betterland Foods
- Motif FoodWorks
- The EVERY Company
- Nature’s Fynd
Terminology to Avoid
The Non-GMO Project tracks GMO 2.0 developments and has a newsletter in which it shares alerts about new GMOs. It warns that the following terms may signal that products contain new GMOs:
- Animal-free dairy proteins
- Bioactive
- Biodesigned
- Bioengineered
- Bioidentical
- Biotechnology/product of biotechnology
- Breast milk proteins
- Determined to be non-GMO by USDA
- Engineered yeast
- Made with yeast, just like beer
- Non-transgenic
- Not regulated by the U.S. government, so not GMO
- Self-made non-GMO claims
- Synbio or synthetic biology
A Solari Report interview, “Untruth in Labeling with Dr. Sina McCullough,” offers many additional suggestions on how to navigate the growing lack of transparency in food labeling and the proliferation of chemical additives.260
In the U.S., insects are not seen as a novel food. If a company wishes to sell them to consumers, they must follow FDA standards and breed them for human consumption. The insects need to be mentioned on the ingredient list, using both the common name and the scientific name.261
For more information, follow and support the following watchdog and educational organizations:
- Non-GMO Project: Certifies non-GMO products, tracks developments in GMO 2.0
- Organic Insider: Reports on important trends in the organic sector
- Center for Food Safety: Informs the public and litigates for safe foods and adequate labeling
- Weston A. Price Foundation: Offers a worldwide network of local chapters to help you find healthy local food and inform you about nutrient-dense, traditional diets
- Farm-to-Consumer Legal Defense Fund: Protects the rights of farmers and consumers to engage in direct commerce
- Organic Consumers Association: Informs and protects consumers of organic products
The EU
In the EU, as of November 2022, no dossiers had been submitted for synthetic meat or lab-fermented food ingredients. GMOs are allowed on the market but are strictly regulated, for now. Farmers are allowed to import GMOs to feed cattle.
That precision-fermented products are allowed on the EU market, without the knowledge of the public at large, is of concern. There is no easy way to access an overview of approved substances and the products that contain them. The EFSA provides a database that lists the substances and “foods” that it has approved, but these contain mostly Latin and chemical names that are unintelligible for laypersons. To get an impression of how normalized the application of precision fermentation in food is, FrieslandCampina, a large international dairy processor located in the Netherlands, “started using precision fermentation in 2016 to produce human milk oligosaccharides. Since then, the food industry’s use of technology has flourished.”184
For the time being, GMOs are not allowed under the organic label. However, an international lobby is pushing Brussels to deregulate the new GMOs.97,98 From leaked documents, it has become clear that the European Commission has included the full deregulation of new genomic techniques (NGTs) in its policy scenarios. This would result in the introduction of NGTs into the market without testing, tracking and tracing, or labeling. The new GMOs would also be allowed under the EU’s organic label. In the second quarter of 2023, the Commission will publish its policy proposal.
Under the EU’s novel food policy, it has also approved five insects for human consumption—mealworms and crickets in different forms—and one nanomaterial, “iron hydroxide adipate tartrate,” that can be added to supplements reportedly to increase bioavailability.262,263
In the EU, organizations worth supporting include GM Watch (reporting on worldwide developments and research with regard to GMOs) and Demeter (a global certification scheme for biodynamic organic agriculture that is committed to keeping all GMOs out of their products).
Global Resources
Useful resources include:
- The Genetically Engineered Food Labeling Laws Map (Center for Food Safety)264
- The Genetic Literacy Project (sponsored by Monsanto and Bayer), which offers a global map explaining the status of each country’s regulations for “human and agricultural gene editing and gene drives”265
- The National Health Federation, which, since 2022, has had a seat at Codex Alimentarius and advocates for people’s rights to normal, safe and healthy foods. Two Solari Reports with National Health Federation founder Scott Tips and Corey Lynn’s article about the “new global diet” provide excellent background on Codex.150,266,267
References
- Neil Stephens, Emma King, and Catherine Lyall. “Blood, meat, and upscaling tissue engineering: promises, anticipated markets, and performativity in the biomedical and agri-food sectors.” BioSocieties. 2018;13:368-388.
- Corey Lynn. “Lab grown meat to hit U.S. in 2022, backed by FDA & USDA, along with ‘Smarter Food Safety Blueprint’ and traceability all underway.” Corey’s Digs, Sept. 22, 2021.
- Jeanette Settembre. “Bill Gates says rich countries should be eating 100% synthetic beef.” Fox News, Feb. 16, 2021.
- Seeding Radical Change: 2021 Year in Review. Good Food Institute, 2021.
- “New Harvest: We are the global nonprofit building the field of cellular agriculture.”
- Elze van Hamelen. “‘One Health’ – Where biosecurity meets Agenda 2030.” Global Research, Aug. 30, 2022.
- Jill Ettinger. “Jeff Bezos, Richard Branson, and Bill Gates lead $90 million investment in vegan meat.” Live Kindly, 2019.
- Neil Stephens, Alexandra E. Sexton, and Clemens Driessen. “Making sense of making meat: key moments in the first 20 years of tissue engineering muscle to make food.” Front Sustain Food Syst. 2019;3:45.
- Amelia Lucas. “Investors bet on meat grown in a lab as interest in plant-based foods cools.” CNBC, Jul. 16, 2022.
- Tom Brennan et al. “Cultivated meat: out of the lab, into the frying pan.” McKinsey & Company, Jun. 16, 2021.
- M. Shahbandeh. “Meat industry value worldwide in 2021 and forecast for 2022 and 2027.” Statista, Aug. 19, 2022.
- Walter G. Johnson, Andrew Maynard, and Sheril Kirshenbaum. “Would you eat ‘meat’ from a lab? Consumers aren’t necessarily sold on ‘cultured meat.’” The Conversation, Aug. 23, 2018.
- Brian Kateman. “Plant-based meat is running out of steam, but it’s still the future.” Fortune, Apr. 11, 2022.
- Emily Soice and Jeremiah Johnston. “Immortalizing cells for human consumption.” Int J Mol Sci. 2021;22(21):11660.
- Antony Froggatt and Laura Wellesley. “Meat analogues: considerations for the EU.” Chatham House, the Royal Institute of International Affairs, Feb. 19, 2019.
- Michael Siegrist and Christina Hartmann. “Consumer acceptance of novel food technologies.” Nature Food. 2020;1:343-350.
- Marc Cervera. “JBS shuts down its US alternative meat operation as market turns on plant-based.” Food Ingredients First, Oct. 4, 2022.
- 2021 State of the Industry Report: Fermentation: Meat, seafood, eggs, and dairy. Good Food Institute, 2022.
- 2021 State of the Industry Report: Cultivated meat and seafood. Good Food Institute, 2022.
- 2021 State of the Industry Report: Plant-based meat, eggs, seafood, and dairy. Good Food Institute, 2022.
- “Alternative protein.” Oslo Dealroom, Nov. 21, 2022.
- Sam Panzer. “FoodTech IPOs and SPACs explained: how it works, who’s doing it and why?” FoodHack, Jun. 21, 2021.
- Matthieu Vincent. “The crazy phenomenon around FoodTech SPACs.” DigitalFoodLab, Jul. 8, 2021.
- Riley de León. “Bill Gates-backed Ginkgo Bioworks going public via $15 billion SPAC.” CNBC, May 11, 2021.
- Megan Poinski. “Moolec Science to go public through SPAC merger.” Food Dive, Jun. 16, 2022.
- Tom Philpott. “Is lab meat about to hit your dinner plate?” Mother Jones, Aug. 2, 2021.
- Karen Gilchrist. “This multibillion-dollar company is selling lab-grown chicken in a world-first.” CNBC Make It, Mar. 1, 2021.
- Janet Forgrieve. “JUST Egg to star in new Whole Foods breakfast sandwiches.” Forbes, Dec. 19, 2019.
- Max Goldberg. “The playbook for GMO 2.0 is going exactly to plan, brands step in to combat it.” Organic Insider, Jun. 8, 2022.
- “New GMOs.” Non-GMO Project. Accessed Oct. 3, 2022.
- Winston Churchill. “Fifty years hence, 1931.” America’s National Churchill Museum, Westminster College. Accessed Jan. 19, 2023.
- Chase Purdy. “The idea for lab-grown meat was born in a prisoner-of-war camp.” Quartz, Sept. 24, 2017.
- “‘Godfather of cultured meat’ Willem van Eelen passes away at 91.” New Harvest, Apr. 8, 2015, updated Oct. 4, 2021.
- “Cultured meat – a timeline.” Cultured Meats Australia, Jul. 9, 2018.
- Isha Datar. “New Harvest, the nonprofit nucleus of cellular agriculture, is in crisis. Emergency town hall on 6/10.” Effective Altruism Forum, Jun. 2, 2022.
- “New Harvest.” Wikipedia. Accessed Nov. 15, 2022.
- Alok Jha. “Google’s Sergey Brin bankrolled world’s first synthetic beef hamburger.” The Guardian, Aug. 5, 2013.
- Alan Boyle. “Google’s Sergey Brin explains why he paid $330,000 for lab burger.” NBC, Aug. 5, 2013.
- The Good Food Institute: Strategic Plan v.7, September 2021. Good Food Institute, September 2021.
- “About GFI.” Good Food Institute. Accessed Nov. 15, 2022.
- “The Good Food Institute.” Wikipedia. Accessed Nov. 15, 2022.
- “Transcript: Ezra Klein interviews Holden Karnofsky.” The New York Times, the Ezra Klein Show, Oct. 5, 2021.
- Tomio Geron. “Top startup incubators and accelerators: Y Combinator tops with $7.8 billion in value.” Forbes, Apr. 30, 2012.
- Delle Chan. “Israel is a fake meat powerhouse.” Wired, Apr. 6, 2021.
- “Is lab-grown meat halal?” The Halal Times, Feb. 2, 2022.
- Caline Malek. “Could lab-grown meat be in Saudi Arabia’s future?” Arab News, Mar. 5, 2019.
- Aryn Baker. “The cow that could feed the planet.” TIME, Nov. 2, 2021.
- “Eat Just & Qatar Free Zones Authority partner on Middle East sustainable food hub.” Business Wire, Aug. 31, 2021.
- “China includes lab-grown meats in its agricultural five-year plan.” China Underground, Jan. 28, 2022.
- WION Web Team. “‘Lab-grown meat’: China’s 5-year agricultural plan with ‘future foods.’” WION, Feb. 2, 2022.
- Food Barons 2022: Crisis Profiteering, Digitalization and Shifting Power. ETC Group, Sept. 7, 2022.
- Sally Ho. “Nearly 30% of world’s biggest food retailers are betting on alternative protein: report.” Green Queen, Oct. 6, 2021.
- Isha Datar. “Launching OpenCellAg.com.” New Harvest, Aug. 5, 2022.
- “Nestlé explores emerging technologies for cultured meat.” Nestlé, Jul. 13, 2021.
- Isha Datar. “What movement building means to us.” New Harvest, Dec. 8, 2021 (updated Dec. 14, 2021).
- “Netherlands to make biggest ever public investment in cellular agriculture.” Good Food Institute, Apr. 14, 2022.
- “Over ons.” Cellulaire Agricultuur Nederland. Accessed Dec. 14, 2022.
- “Gene drives.” EcoNexus. Accessed Jan. 25, 2023.
- Anju Verma, Megha Verma, and Anchal Singh. “Animal tissue culture principles and applications.” Animal Biotechnology. 2020:269-293.
- “About the American Association of Tissue Banks.” AATB. Accessed Nov. 25, 2022.
- Brad N. Taylor. “Streamlining regulatory compliance for cell culture media.” Nucleus Biologics, Jul. 27, 2022.
- Sound Choice Pharmaceutical Institute, accessed Dec. 7, 2022.
- Sound Choice Pharmaceutical Institute, accessed Dec. 9, 2022.
- Joe Fassler. “Lab-grown meat is supposed to be inevitable. The science tells a different story.” The Counter, Sept. 22, 2021.
- “PFIZER LEAKS: Whistleblower goes on record, reveals internal emails from chief scientific officer & senior director of worldwide research discussing COVID vaccine … ‘We want to avoid having the information on the fetal cells floating out there.’” Project Veritas, Oct. 6, 2021.
- Caryn Lipson. “Aborted fetal cells and vaccines—a scandal much bigger than Pfizer’s whistleblower ever imagined.” Oct. 18, 2021.
- Christian Hacking. “What the HEK?!” CBR UK, Feb. 9, 2021.
- Jon Rappoport. “The abortion culture.” Jon Rappoport’s Blog, Oct. 27, 2021.
- Thomas Seidler. “Vaccines using fetal tissue: 12 faulty assumptions.” LifeSiteNews, Apr. 1, 2021.
- “RFK, Jr. discusses aborted fetal DNA and vaccines with Dr. Theresa Deisher.” Children’s Health Defense, Jun. 17, 2020.
- Monica Seeley. “Exploring the dark world of vaccines and fetal tissue research: Part 1.” Catholic World Report, May 17, 2021.
- Monica Seeley. “Exploring the dark world of vaccines and fetal tissue research: Part 2.” Catholic World Report, May 26, 2021.
- “Fetal tissue in vaccines, studies on orphans—confirmed by Stanley Plotkin under oath.” Children’s Health Defense, Sept. 26, 2019.
- “Live aborted fetus experimenting.” Accessed Dec. 9, 2022.
- “Fetal tissue panel issues final report.” AAMC, Jan. 5, 2017.
- Select Investigative Panel of the Energy & Commerce Committee. Select Investigative Panel: Final Report. U.S. House of Representatives, December 30, 2016.
- Sam Dorman. “Doctors say Pitt statements point to possibility organs extracted from live fetuses; school denies charge.” Fox News, Aug. 11, 2021.
- David Daleiden. “University of Pittsburgh won’t explain its Planned Parenthood ties.” Newsweek, May 26, 2021.
- “BREAKING: University of Pittsburgh ADMITS hearts beating while harvesting aborted infants’ kidneys.” The Center for Medical Progress, Aug. 4, 2021.
- Cade Hildreth. “How many billions are spent on stem cell funding? (NIH spends $1.5B/year).” BioInformant, Sept. 20, 2016.
- Matej Mikulic. “Total stem cell research funding by the National Institutes for Health (NIH) from FY 2013 to FY 2018.” Statista, Jul. 13, 2017.
- “History of stem cell use.” University of Nebraska Medical Center. Accessed Nov. 16, 2022.
- “Stem cell basics.” National Institutes of Health. Accessed Jan. 24, 2023.
- Aleszu Bajak. “Will embryonic stem cells ever cure anything?” MIT Technology Review, Aug. 12, 2016.
- “Fetal tissue sales.” Snopes, Jul. 24, 2015.
- “Setting the stage: Fetal research, fetal tissue research, and historical timeline of regulation and legislation.” In Fetal Research and Applications: A Conference Summary. Institute of Medicine (US) Conference Committee on Fetal Research and Applications. Washington, DC: National Academies Press (US), 1994.
- Maggie Fox. “Planned Parenthood video: Why use tissue from aborted fetuses?” NBC News, Jul. 17, 2015 (updated Sept. 25, 2018).
- Tara Sander Lee et al. “Human fetal tissue from elective abortions in research and medicine: science, ethics, and the law.” Issues Law Med. 202035(1):3-61.
- “Tissue engineering and regenerative medicine.” National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health. Accessed Nov. 15, 2022.
- Gianluca Sampogna, Salman Yousuf Guraya, and Antonello Forgione. “Regenerative medicine: historical roots and potential strategies in modern medicine.” J Microsc Ultrastruct. 2015;3(3):101-107.
- Greg Gillispie. “Tissue engineering and regenerative medicine in longevity.” Lifespan.io, Sept. 9, 2020.
- Ricarda A. Steinbrecher. “Genetic engineering in plants and the ‘new breeding techniques (NBTs)’: inherent risks and the need to regulate.” EcoNexus, December 2015.
- “Gene-edited hornless cattle: flaws in the genome overlooked.” GM Watch, Aug. 9, 2019.
- “Biotech lobby’s push for new GMOs to escape regulation.” Corporate Europe Observatory, Feb. 2, 2016.
- “Farm to Fork strategy for a fair, healthy and environmentally-friendly food system.” European Commission. Accessed Jan. 25, 2023.
- F. William Engdahl. “Farm to Fork: how the EU and the Davos cabal plan to control agriculture.” Sept. 29, 2021.
- Elze van Hamelen. “Als de EU gmo’s dereguleert, zullen vele landen volgen” – interview with Claire Robinson from GM Watch. De Andere Krant, Dec. 2, 2022.
- Claire Robinson. “EU Commission’s secret policy scenarios show full GMO deregulation on the cards.” GM Watch, Jul. 22, 2022.
- Michael Chui et al. The Bio Revolution: Innovations Transforming Economies, Societies, and Our Lives. McKinsey Global Institute, May 2020.
- “What is synbio?” Syn-Bio.Org. Accessed Nov. 16, 2022.
- “Synthetic biology.” Wikipedia. Accessed Oct. 16, 2022.
- Christopher Preston. “From synthetic life to engineering the climate—how we’re learning to manage the Earth.” World Economic Forum, May 17, 2018.
- Benjamin Wolfson. “DARPA and the future of synthetic biology.” O’Reilly, Dec. 6, 2017.
- John Cumbers and Matthew Kirshner. “If biology can build it, they will come: Ginkgo Bioworks is laying the foundation for the $4 trillion bioeconomy.” SynBioBeta, 2019.
- Antje Lorch. “Synthetic biology: submission to the CBD.” EcoNexus, October 2011.
- “New GMO alert: molecular farming.” Non-GMO Project, Sept. 2, 2022.
- Stacy Malkan. “Bill Gates’ radical menu for food systems: ultra-processed foods, patents, monocrops.” U.S. Right to Know, May 26, 2021.
- “Biotech firm Ginkgo to merge with Harry Sloan-led SPAC in $17.5 bln deal.” Reuters, May 11, 2021.
- Riley Griffin. “Ginkgo is trying to detect future man-made biological threats.” Bloomberg, Oct. 17, 2022.
- Jaydee Hanson and Julia Ranney. “Is lab-grown meat healthy and safe to consume?” Center for Food Safety, Sept. 20, 2020.
- Jacob Reiss, Samantha Robertson, and Masatoshi Suzuki. “Cell sources for cultivated meat: applications and considerations throughout the production workflow.” Int J Mol Sci. 2021;22(14):7513.
- David Humbird. “Scale-up economics for cultured meat.” Biotechnol Bioeng. 2021;118(8):3239-3250.
- “Deep dive: cultivated meat cell lines.” Good Food Institute. Accessed Jan. 26, 2023.
- Matt Reynolds. “The hunt for the master cow that will feed the world.” Wired, May 25, 2021.
- Neil Stephens, Lucy Di Silvio, et al. “Bringing cultured meat to market: technical, socio-political, and regulatory challenges in cellular agriculture.” Trends Food Sci Technol. 2018;78:155-166.
- “Kerafast’s partnership with the Good Food Institute links researchers to cell lines.” Kerafast, Jul. 13, 2021.
- Nicole Gleichmann. “Which cell culture media is right for you?” Technology Networks, Mar. 29, 2022.
- “Fetal bovine serum market expected to reach USD 1,401.23 million in value by 2030: Straits Research.” GlobeNewswire, May 2, 2022.
- Nick Thieme. “The gruesome truth about lab-grown meat.” Slate, Jul. 11, 2017.
- Kimberly J. Ong et al. “Food safety considerations and research priorities for the cultured meat and seafood industry.” Compr Rev Food Sci Food Saf. 2021;20(6):5421-5448.
- Avery Parkinson. “Breaking down the Good Food Institute’s Essential 8 culture media analysis.” New Harvest, Jan. 13, 2020.
- “Tiamat Sciences – Using plants in place of bioreactors to meet the future needs of cellular agriculture.” Protein Report, Jul. 15, 2020.
- Sghaier Chriki and Jean-François Hocquette. “The myth of cultured meat: a review.” Front Nutr. 2020;7:7.
- “TEA of cultivated meat. Future projections for different scenarios.” CE Delft, November 2021.
- “What is Cell Ag?” New Harvest. July 2022.
- Claire Bomkamp et al. “Scaffolding biomaterials for 3D cultivated meat: prospects and challenges.” Adv Sci (Weinh). 2022;9(3):e2102908.
- Mitch and Hunter. “Matrix Meats and the world of 3D scaffolding.” Lab-Grown Meat, Aug. 20, 2021.
- Electrospun Polymer Fibers for Cultured Meat Production. United States Patent Application 20200245658. Free Patents Online, Aug. 6, 2020.
- Zhongyang Liu et al. “Recent advances on magnetic sensitive hydrogels in tissue engineering.” Front Chem. 2020;8:124.
- “Deep dive: Cultivated meat scaffolding.” Good Food Institute. Accessed Dec. 11, 2022.
- “About nanotechnology.” National Nanotechnology Initiative. Accessed Jan. 29, 2023.
- Ulrike Granögger. “Future Science Series: Medical Nanobots—Implications of the Wave Genome, Part II.” Solari Report, Jun. 10, 2021.
- “Deep dive: Cultivated meat bioprocess design.” Good Food Institute. Accessed Dec. 11, 2022.
- “Food safety advocates call for regulation and transparent labeling of cell-cultured lab ‘meats.’” Center for Food Safety, Dec. 3, 2021.
- Anna Starostinetskaya. “Singapore becomes first country in the world to allow sale of cell-based meat.” Veg News, Dec. 2, 2020.
- “Reducing the price of alternative proteins.” Good Food Institute. Accessed Nov. 22, 2022.
- “New studies further the case for cultivated meat over conventional meat in the race to net-zero emissions.” Good Food Institute. Accessed Nov. 2, 2022.
- Amy Fleming. “What is lab-grown meat? How it’s made, environmental impact and more.” Science Focus, Jun. 9, 2022.
- María Ignacia Rodríguez Escobar et al. “Analysis of the cultured meat production system in function of its environmental footprint: current status, gaps and recommendations.” Foods. 2021;10(12):2941.
- Joshua Hadi and Gale Brightwell. “Safety of alternative proteins: technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein.” Foods. 2021;10(6):1226.
- Yadira Tejeda-Saldana. “New Harvest publishes cell ag’s first safety paper.” New Harvest, Oct. 13 2021.
- Children’s Health Defense Team. “Study: ‘Major differences’ between grass-fed and plant-based meat, despite similar nutrition labels.” The Defender, Aug. 24, 2021.
- “Highly nutritious meat substitutes on the market cannot be absorbed by the human body, study flags.” Nutrition Insight, Dec. 9, 2022.
- Claire Robinson. “Rat feeding study suggests the Impossible Burger may not be safe to eat.” GM Watch, Sep. 20, 2022.
- “IPCC: Plant-based and cultivated meat can play a critical role in halving global emissions by 2030.” Good Food Institute. Accessed Jan. 26, 2022.
- Colin Todhunter. “The Netherlands: template for ecomodernism’s brave new world?” Off-Guardian, Dec. 12, 2022.
- Robert F. Kennedy, Jr. “Bill Gates and neo-feudalism: a closer look at farmer Bill.” The Defender, Feb. 4, 2021.
- F. William Engdahl. “Now the organized takedown of global fertilizer supply?” Nov. 12, 2021.
- Kit Knightly. “Lab-grown meat & nuclear yeast vats: COP27 reignites the war on food.” Off-Guardian, Nov. 13, 2022.
- Corey Lynn. “Inside Codex with Scott Tips: new global food diet – insects, rats and dogs.” Corey’s Digs, Oct. 13, 2022.
- Corey Lynn. “NEW controlled food system is now in place and they will stop at nothing to accelerate their control.” Corey’s Digs, Apr. 27, 2022.
- Corey Lynn. “New “food is medicine” nutrition screening, RX, tracking and control.” Corey’s Digs, Nov. 22, 2022.
- F. William Engdahl. “War on global agriculture: the unsustainable ‘sustainable’ UN Agenda 2030.” Nov. 30, 2022.
- “Food and Agriculture for Sustainable Transformation Initiative: FAST.” Food and Agriculture Organization, 2022.
- Daphne Ewing-Chow. “Groundbreaking nutrition-climate initiative launched at COP27. Forbes, Nov. 12, 2022.
- “The Future of Protein Action Community.” World Economic Forum. Accessed Jan. 26, 2023.
- Global Coordinating Secretariat | WEF-NL en de toekomst van voedsel. Vraagtekens. Jul. 9, 2022.
- “Global center for food innovation comes to the Netherlands.” Oost NL, Jan. 29, 2021.
- Elze van Hamelen. “The nation-state has become an empty shell” | Interview Patrick M. Wood for De Andere Krant. Odysee, Sep. 12, 2022.
- “Horizon Europe.” European Commission, April 2021.
- “‘Farm to Fork’ strategy: Striving for healthy and sustainable food.” European Parliament, June 2020.
- “Wetenschappers pleiten voor toestaan nieuwe veredelingstechnieken in Europa’s biologische landbouw.” WUR, Apr. 23, 2021.
- Gerardo Fortuno. “Beyond Farm to Fork: the ‘agricultural’ side of EU’s biodiversity strategy.” Euractiv, Mar. 4, 2021.
- Climate Solutions.” USDA. Accessed Jan. 26, 2023.
- “Executive Order on Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy.” White House, Sep. 12, 2022.
- “Executive order is a massive push toward further privatization of U.S. food supply.” Non-GMO Project, Sep. 4, 2022.
- “One Health.” WHO, Sep. 21, 2017.
- Elze van Hamelen. “COVID measures: biggest ‘social conformity event’ in history. Corona policy was aimed at ‘changing behavior,’ not at improving health.” Global Research. Sep. 21, 2022.
- Elze van Hamelen. “Gedragssturing: Burger moet aan insecten en kweekvlees.” De Andere Krant, Oct. 23, 2022.
- “Haarlem becomes first city in the world to ban meat advertising.” Dutch News, Sep. 5, 2022.
- “Over voedseleducatie.” Smaaklessen. Accessed Nov. 11, 2022.
- “Meelworm op het menu in lespakket honderd basisscholen in Overijssel.” RTV Oost, Oct. 27, 2022.
- Reset the Table: Meeting the Moment to Transform the U.S. Food System. Rockefeller Foundation, Jul. 28, 2020.
- “The Rockefeller Foundation invests $4.6 million to scale Food Is Medicine initiatives in U.S.” The Rockefeller Foundation, Nov. 30, 2022.
- “NIH awards $170 million for precision nutrition study.” NIH, Jan. 20, 2022.
- Jennifer Marston. “BREAKING: FAIRR & Good Food Institute launch sustainability reporting frameworks for alternative protein.” AgFunder News, Sep. 8, 2022.
- Gabriella Hoffman. “SEC’s new ESG rule hurts America’s small farms and ranches.” RealClear Energy, Jun. 22, 2022.
- “Food made with cultured animal cells.” FDA, content current as of Nov. 16, 2022.
- “FDA completes first pre-market consultation for human food made using animal cell culture technology.” FDA, Nov. 16, 2022.
- Jaydee Hanson. “Statement on FDA’s first-ever approval of lab grown chicken.” Center for Food Safety, Nov. 17, 2022.
- Flora Southey. “Why is EFSA yet to receive a novel food dossier on cell-based meat?” Food Navigator, Oct. 31, 2022.
- Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed (Text with EEA relevance).
- “Safety of 2’-0-fucosyllactose as a novel food ingredient pursuant to Regulation (EC) No 258/97.” EFSA Journal. 2015;13(7):4184.
- Marc Cervera. “FrieslandCampina Ingredients expands beyond traditional dairy with microbial fermentation collaboration.” Food IngredientsFirst, Jan. 10, 2023.
- Jing Pan. “The EU just approved insects for human consumption with plenty of ‘environmental benefits’—are mealworms the low-cost, sustainable answer to soaring meat prices? Yahoo!finance, Jan. 27, 2023.
- De Andere Krant, Jan. 28, 2023.
- “Novel food regulations around the world.” GFI Asia Pacific, 2022.
- Anne-Laure Robin. “Cultured meat: navigating the regulations in the EU, USA and China.” Leatherhead Food Research, 2021.
- “Cellular Agriculture.” Center for Rule-making Strategies-Japan. Accessed Dec. 9, 2022.
- “USDA seeks comments on the labeling of meat and poultry products derived from animal cells.” USDA, Sep. 2, 2021.
- “Food safety advocates call for regulation and transparent labeling of cell-cultured lab ‘meats.’” Center for Food Safety, Dec. 3, 2021.
- Emily Monaco. “French government moves to ban words like ‘milk’ and ‘meat’ on vegan good labels.” Organic Authority, May 7, 2019.
- Lauren Stone. “GFI, Codex, and building global unity around alternative proteins.” Good Food Institute, Jan. 28, 2022.
- Vinícius Gallon. “Codex Alimentarius Commission grants GFI observer status.” Good Food Institute, Feb. 24, 2021.
- Katie O’Leary. “15 VC firms investing in pet technology.” Pitchbook, Feb. 24, 2022.
- “Agronomics reveals cultivated meat pet food venture Good Dog Food.” Vegconomist, Mar. 22, 2022.
- “Cell-cultured meat goes to the dogs.” The Counter, Feb. 2, 2021.
- Jade Scipioni. “Billionaire Peter Thiel backs vegan pet food startup developing lab-grown mouse meat.” Fox, Jul. 30, 2018.
- Louisa Burwood-Taylor. “Protix raises $50m in largest insect farming investment on record.” AgFunder News, Jun. 14, 2017.
- Remigiusz Gałęcki and Rajmund Sokól. “A parasitological evaluation of edible insects and their role in the transmission of parasitic diseases to humans and animals.” PloS One. 2019;14(7):e0219303.
- “Transmission of parasitic diseases.” Centers for Disease Control and Prevention. Accessed Jan. 29, 2023.
- Kimberly Richardson. “Our pets are part of the climate problem. These tips can help you minimize their carbon pawprints.” CNN, Sep. 27, 2022.
- Jennifer Hahn. “Ouroboros Steak grow-your-own human meat kit is ‘technically’ not cannibalism.” Dezeen, Nov. 13, 2020.
- Jenn Harris. “Ellen DeGeneres salami? One company’s quest to make meat from celebrity tissue samples.” Los Angeles Times, Mar. 5, 2014.
- James Felton. “A company wants to turn celebrities into lab-grown salami—and it’s perfectly possible.” IFLScience, Jan. 18, 2022.
- Wesley J. Smith. “Dawkins wants to eat human ‘meat.’” National Review, Mar. 6, 2018.
- “Scientist proposes cannibalism to ‘save the climate’ on Swedish TV.” Vigilant Citizen, Sep. 11, 2019.
- “Netflix is losing subscribers in the US: the untold reason.” Vigilant Citizen, Jul. 18, 2019.
- “The meaning of Netflix’s ‘Brand New Cherry Flavor’: a celebration of occult Hollywood.” Vigilant Citizen, Sep. 21, 2021.
- Jonathon Van Maren. “New York Times says cannibalism ‘has a time and a place’ in new low for our cultural elites.” LifeSiteNews, Jul. 25, 2022.
- Kathryn Jean Lopez. “Cannibalism chic?” National Review, Jul. 25, 2022.
- William A. Haseltine. “Unregulated stem cell clinics endanger patients and limit research.” Forbes, Nov. 13, 2021.
- Patrick Wood. “Biodiversity: the genetic takeover of all living things.” Technocracy News, Feb. 22, 2022.
- Patrick Wood. “Global blueprint exposed: the takeover of all genetic material on earth.” Technocracy News, Oct. 24, 2022.
- Whitney Webb. “Wall Street’s takeover of nature advances with launch of new asset class.” Unlimited Hangout, Oct. 13, 2021.
- Sara N. Garcia, Benny I. Osburn, and Michele T. Jay-Russell. “One Health for food safety, food security, and sustainable food production.” Front Sustain Food Syst. 2020;4.
- Peter Gerner-Smidt et al. “Whole genome sequencing: bridging One-Health surveillance of foodborne diseases.” Front Public Health. 2019;7:172.
- Marion Bordier et al. “Characteristics of One Health surveillance systems: a systematic literature review.” Prev Vet Med. 2020;181:104560.
- “Edible vaccine.” ScienceDirect. Accessed Dec. 18, 2022.
- Jules Bernstein. “Grow and eat your own vaccines?” University of California, Sep. 16, 2021.
- Claire Desfrançois, Rachel Auzély, and Isabelle Texier. “Lipid nanoparticles and their hydrogel composites for drug delivery: a review.” Pharmaceuticals (Basel). 2018;11(4):118.
- NIH.gov. “Advances on magnetic sensitive hydrogels in tissue engineering.” Principia Scientific International, May 14, 2021.
- Whitney Webb. “Moderna: a company “in need of a Hail Mary.” Unlimited Hangout, Oct. 7, 2021.
- “Injectable body sensors take personal chemistry to a cell phone closer to reality.” Profusa, Mar. 19, 2018.
- Makia Freeman. “Hydrogel biosensor: implantable nanotech to be used in COVID vaccines?” David Icke, Sep. 3, 2020.
- Erin Brodwin. “The company behind America’s favorite hummus has funded an under-the-radar effort to make lab-grown steak.” Business Insider, May 3, 2018.
- “The legacy.” The Roslin Institute. Accessed Nov. 21, 2022.
- “From Dolly the sheep to cultivated meat: how Roslin Technologies is facilitating scale-up success for the cultivated meat industry.” Future Planet Capital on Linkedin, Mar. 30, 2022.
- “Dean Kamen.” Wikipedia. Accessed Nov. 15, 2022.
- Jessica Hamzelou. “Inside the billion-dollar meeting for the mega-rich who want to live forever.” MIT Technology Review, Nov. 16, 2022.
- Stephanie Wallis. “Could cultivated meat fast-track regenerative medicine?” TED, Jan. 16, 2020.
- Ruth Purcell. “Cellular Agriculture Australia: right place, right time, right tech.” CellAgri, Dec. 13, 2021.
- “Nanofiber Solutions, LLC.” SBIR STTR: America’s Seed Fund. Accessed November 2022.
- Joe McGauley. “Amazon’s CEO is on a quest to stop aging.” Thrillist, Jan. 27, 2017.
- Jason Mast. “In blow to anti-aging field, Unity Biotechnology fails first major study, cuts lead program.” Endpoint News, Aug. 17, 2020.
- Antonio Regalado. “How scientists want to make you young again.” MIT Technology Review, Oct. 25, 2022.
- “Meatable raises $47 million Series A to continue scaling trajectory and expand its product portfolio with beef.” Meatable, Mar. 23, 2021.
- “A look at Peter Thiel’s biotechnology investments.” Fight Aging, Mar. 16, 2015.
- Paul Tullis. “Are you rich enough to live forever?” Town and Country Magazine, Mar. 30, 2019.
- Edward Devlin. “Agronomics launches Roslin Technologies joint venture to develop lab grown pet food.” The Grocer, Mar. 22, 2022.
- Jason G. Matheny. “Reducing the risk of human extinction.” Risk Anal. 2007;27(5):1335-1344.
- Valerie Richardson. “Mini ice age likely from 2030 to 2040, European scientists say.” Washington Times, Jul. 12, 2015.
- John Roach. “Earth’s magnetic field is fading.” National Geographic, Sep. 9, 2004.
- Pam Wright. “Earth’s atmosphere is losing oxygen at an accelerated rate, study says.” The Weather Channel, Sep. 26, 2016.
- James Clayton. “SpaceX: Can meat be grown in space?” BBC, Apr. 25, 2022.
- “ESA investigates cultured meat as novel space food.” European Space Agency, Jun. 1, 2021.
- Pete Kennedy. “Finding sources of fresh food.” Solari Report, Feb. 4, 2022.
- Solari Food Series.
- Solari Team. “Food resilience action for family and community: gardening.” Solari Report, Jul. 5, 2019.
- Stephen Scott. “Russian dacha gardening – homescale agriculture feeding everyone.” Underwood Gardens, Apr. 15, 2013.
- Elze van Hamelen. Interview with Dan Kovalic on the elections in Nicaragua for De Andere Krant. YouTube, December 2021.
- “Be part of the solution.” Square Foot Gardening Foundation.
- Breanne Deppisch. “Netherlands to buy out and close 3,000 farms to meet climate goals.” Washington Examiner, Nov. 29, 2022.
- Tyler Durden. “Bill Gates becomes America’s largest farmland owner while ‘Great Reset’ says future is ‘no private property.’” Zero Hedge, Jan. 16, 2021.
- “Report: Terminology proposed in USDA GMO labeling rule would confuse and mislead consumers.” Non-GMO Project, Jul. 2, 2018.
- “What’s behind the USDA organic seal?” USDA, July 2021.
- Max Goldberg. “The first step for GMOs to be allowed in organic is public discussion—which is now happening.” Organic Insider, Nov. 16, 2022.
- “GMOs 2.0/synthetic biology.” The Organic & Non-GMO report, October 2022.
- Pete Kennedy. “Food Series: Untruth in labeling with Dr. Sina McCullough.” Solari Report, Jun. 18, 2022.
- “Legal status of edible insects in some countries.” Bugsolutely, 2022.
- “Approval of third insect as a Novel Food.” Food European Commission, Feb. 10, 2022.
- “Safety of iron hydroxide adipate tartrate as a novel food pursuant to Regulation (EU) 2015/2283 and as a source of iron in the context of Directive 2002/46/EC.” EFSA, Oct. 27, 2021.
- Catherine Austin Fitts. “Codex Alimentarius and the push for central control with Scott Tips.” Solari Report, May 19, 2022.
- Catherine Austin Fitts. “Scott Tips on Codex: the global scheme to control your food.” Solari Report, Jul. 20, 2011.
